Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS
- PMID: 24825863
- PMCID: PMC4110663
- DOI: 10.1182/blood-2014-02-554980
Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS
Abstract
Antiphospholipid syndrome (APS) is defined by thrombosis, fetal loss, and the presence of antiphospholipid antibodies, including anti-β2-glycoprotein-1 autoantibodies (anti-β2GP1) that have a direct role in the pathogenesis of thrombosis in vivo. The cellular targets of the anti-β2GP1 autoantibody/β2GP1 complex in vivo were studied using a laser-induced thrombosis model of APS in a live mouse and human anti-β2GP1 autoantibodies affinity-purified from APS patients. Cell binding of fluorescently labeled β2GP1 and anti-β2GP1 autoantibodies revealed their colocalization on the platelet thrombus but not the endothelium. Anti-β2GP1 autoantibodies enhanced platelet activation, monitored by calcium mobilization, and endothelial activation, monitored by intercellular adhesion molecule-1 expression. When eptifibatide was infused to block platelet thrombus formation, enhanced fibrin generation and endothelial cell activation were eliminated. Thus, the anti-β2GP1 autoantibody/β2GP1 complex binds to the thrombus, enhancing platelet activation, and platelet secretion leads to enhanced endothelium activation and fibrin generation. These results lead to a paradigm shift away from the concept that binding of the anti-β2GP1 autoantibody/β2GP1 complex activates both endothelial cells and platelets toward one in which activation of platelets in response to anti-β2GP1 autoantibody/β2GP1 complex binding leads to subsequent enhanced endothelium activation and fibrin generation.
© 2014 by The American Society of Hematology.
Figures
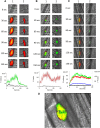
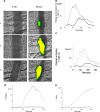
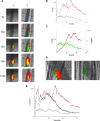
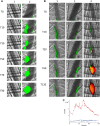
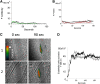
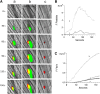
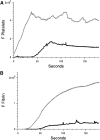
Comment in
-
Platelets as pivot in the antiphospholipid syndrome.Blood. 2014 Jul 24;124(4):475-6. doi: 10.1182/blood-2014-06-576983. Blood. 2014. PMID: 25061169 Free PMC article.
References
-
- de Groot PG, Urbanus RT. The significance of autoantibodies against β2-glycoprotein I. Blood. 2012;120(2):266–274. - PubMed
-
- Willis R, Harris EN, Pierangeli SS. Pathogenesis of the antiphospholipid syndrome. Semin Thromb Hemost. 2012;38(4):305–321. - PubMed
-
- Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011;7(6):330–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

