A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways
- PMID: 24825919
- PMCID: PMC4138219
- DOI: 10.1126/scisignal.2005261
A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways
Abstract
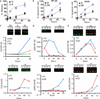
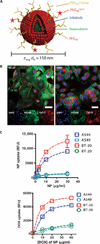
Exposure to the EGFR (epidermal growth factor receptor) inhibitor erlotinib promotes the dynamic rewiring of apoptotic pathways, which sensitizes cells within a specific period to subsequent exposure to the DNA-damaging agent doxorubicin. A critical challenge for translating this therapeutic network rewiring into clinical practice is the design of optimal drug delivery systems. We report the generation of a nanoparticle delivery vehicle that contained more than one therapeutic agent and produced a controlled sequence of drug release. Liposomes, representing the first clinically approved nanomedicine systems, are well-characterized, simple, and versatile platforms for the manufacture of functional and tunable drug carriers. Using the hydrophobic and hydrophilic compartments of liposomes, we effectively incorporated both hydrophobic (erlotinib) and hydrophilic (doxorubicin) small molecules, through which we achieved the desired time sequence of drug release. We also coated the liposomes with folate to facilitate targeting to cancer cells. When compared to the time-staggered application of individual drugs, staggered release from tumor-targeted single liposomal particles enhanced dynamic rewiring of apoptotic signaling pathways, resulting in improved tumor cell killing in culture and tumor shrinkage in animal models.
Copyright © 2014, American Association for the Advancement of Science.
Figures



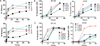
Comment in
-
Combinatorial nanotherapeutics: rewiring, then killing, cancer cells.Sci Signal. 2014 May 13;7(325):pe13. doi: 10.1126/scisignal.2005386. Sci Signal. 2014. PMID: 24825918
References
-
- Weinberg RA. The Biology of Cancer. New York: Garland Science, Taylor & Francis Group LLC; 2007. p. 864.
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. - PubMed
-
- Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nat. Chem. Biol. 2006;2:458–466. - PubMed
-
- Kong DX, Li XJ, Zhang HY. Where is the hope for drug discovery? Let history tell the future. Drug Discov. Today. 2009;14:115–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES015339/ES/NIEHS NIH HHS/United States
- R21-ES020466/ES/NIEHS NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- F32 EB017614/EB/NIBIB NIH HHS/United States
- U54-CA151884/CA/NCI NIH HHS/United States
- 1F32EB017614-01/EB/NIBIB NIH HHS/United States
- R01-ES015339/ES/NIEHS NIH HHS/United States
- U54 CA151884/CA/NCI NIH HHS/United States
- P30-CA14051/CA/NCI NIH HHS/United States
- U54 CA112967/CA/NCI NIH HHS/United States
- R21 ES020466/ES/NIEHS NIH HHS/United States
- U54-CA112967/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

