2-[4-(Piperidin-1-yl)-5H-chromeno[2,3-d]pyrimidin-2-yl]phenol
- PMID: 24826150
- PMCID: PMC3998607
- DOI: 10.1107/S1600536814005625
2-[4-(Piperidin-1-yl)-5H-chromeno[2,3-d]pyrimidin-2-yl]phenol
Abstract
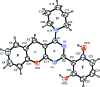
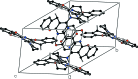
In the title compound, C22H21N3O2, the pyrimidine ring is essentially planar [maximum deviation = 0.018 (2) Å] and forms dihedral angles of 22.70 (8) and 0.97 (7)°, respectively, with the fused benzene ring and the hy-droxy-substituted benzene ring. The piperidine ring has a chair conformation and the pyran ring has a flattened twist-boat conformation. The hy-droxy group was refined as disordered over two sets of sites in a 0.702 (4):0.298 (4) ratio. The disorder corresponds to a rotation of approxomiately 180° about the C-C bond connecting the phenol group to the pyrimidine ring and hence, both the major and minor components of disorder form intra-molecular O-H⋯N hydrogen bonds. In the crystal, pairs of weak C-H⋯π inter-actions form inversion dimers. In addition, π-π inter-actions are observed between the pyrimidine ring and the hy-droxy-substituted benzene ring [centroid-centroid separation = 3.739 (2) Å].
Figures
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Brahmachari, G. & Das, S. (2014). J. Heterocycl. Chem. In the press. 10.1002/jhet.2123.
-
- Bruno, O., Brullo, C., Ranise, A., Schenone, S., Bondavalli, F., Barocelli, E., Ballabeni, V., Chiavarini, M., Tognolini, M. & Impicciatore, M. (2001). Bioorg. Med. Chem. Lett. 11, 1397–1400. - PubMed
-
- Bruno, O., Brullo, C., Schenone, S., Bondavalli, F., Ranise, A., Tognolini, M., Ballabeni, V. & Barocelli, E. (2004). Bioorg. Med. Chem. Lett. 12, 553–561. - PubMed
-
- Duax, W. L. & Norton, D. A. (1975). In Atlas of Steroid Structures, Vol. 1. New York: Plenum.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
