Accrual and recruitment practices at Clinical and Translational Science Award (CTSA) institutions: a call for expectations, expertise, and evaluation
- PMID: 24826854
- PMCID: PMC4116452
- DOI: 10.1097/ACM.0000000000000308
Accrual and recruitment practices at Clinical and Translational Science Award (CTSA) institutions: a call for expectations, expertise, and evaluation
Abstract
Purpose: To respond to increased public and programmatic demand to address underenrollment of clinical translational research studies, the authors examined participant recruitment practices at Clinical and Translational Science Award (CTSA) sites and make recommendations for performance metrics and accountability.
Method: The CTSA Recruitment and Retention taskforce in 2010 invited representatives at 46 CTSAs to complete an online 48-question survey querying accrual and recruitment outcomes, practices, evaluation methods, policies, and perceived gaps in related knowledge/practice. Descriptive statistical and thematic analyses were conducted.
Results: Forty-six respondents representing 44 CTSAs completed the survey. Recruitment conducted by study teams was the most common practice reported (78%-91%, by study type); 39% reported their institution offered recruitment services to investigators. Respondents valued study feasibility assessment as a successful practice (39%); desired additional resources included feasibility assessments (49%) and participant registries (44%). None reported their institution systematically required justification of feasibility; some indicated relevant information was considered prior to institutional review board (IRB) review (30%) or contract approval (22%). All respondents' IRBs tracked study progress, but only 10% of respondents could report outcome data for timely accrual. Few reported written policies addressing poor accrual or provided data to support recruitment practice effectiveness.
Conclusions: Many CTSAs lack the necessary frame work to support study accrual. Recom men dations to enhance accrual include articulating institutional expectations and policy for routine recruitment plan ning; providing recruitment expertise to inform feasibility assessment and recruit ment planning; and developing interdepartmental coordination and integrated informatics infrastructure to drive the conduct, evaluation, and improvement of recruitment practices.
Figures
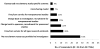
References
-
- English RA, Lebovitz Y, Griffin RB. Transforming Clinical Research in the United States:Challenges and Opportunities: Workshop Summary. Washington, DC: The National Academies Press; 2010. [Accessed March 19, 2014]. Forum on Drug Discovery, Development, and Translation. http://www.nap.edu/openbook.php?record_id=12900. - PubMed
-
- Leshner AI, Terry SF, Schultz AM, Liverman CT, editors. The CTSA Program at NIH: Opportunities for Advancing Clinical and Translational Research. Washington, DC: The National Academies Press; 2013. [Accessed 2013]. http://www.iom.edu/reports/2013/the-ctsa-program-at-nih-opportunities-fo.... - PubMed
-
- Anderson DL. A Guide to Patient Recruitment and Retention. Boston, Mass: Thomson CenterWatch; 2004.
-
- Getz KA, Wenger J, Campo RA, Seguine ES, Kaitin KI. Assessing the impact of protocol design changes on clinical trial performance. Am J Ther. 2008;15(5):450–457. - PubMed
Publication types
MeSH terms
Grants and funding
- UL1 TR001070/TR/NCATS NIH HHS/United States
- UL1 TR000043/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- UL1TR000128/TR/NCATS NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- UL1 TR000436/TR/NCATS NIH HHS/United States
- UL1 TR000071/TR/NCATS NIH HHS/United States
- UL1 TR000424/TR/NCATS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1 TR000083/TR/NCATS NIH HHS/United States
- UL1TR000042/TR/NCATS NIH HHS/United States
- UL1TR000424/TR/NCATS NIH HHS/United States
- UL1TR000064/TR/NCATS NIH HHS/United States
- UL1 TR001111/TR/NCATS NIH HHS/United States
- UL1 TR000090/TR/NCATS NIH HHS/United States
- UL1TR000083/TR/NCATS NIH HHS/United States
- UL1TR000090/TR/NCATS NIH HHS/United States
- UL1 TR000128/TR/NCATS NIH HHS/United States
- UL1TR000043/TR/NCATS NIH HHS/United States
- UL1TR000071/TR/NCATS NIH HHS/United States
- UL1TR000448/TR/NCATS NIH HHS/United States
- UL1TR000436/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

