Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR
- PMID: 24827152
- PMCID: PMC4020785
- DOI: 10.1371/journal.pone.0097257
Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR
Abstract
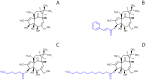
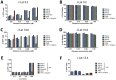
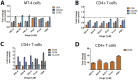
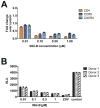
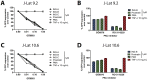
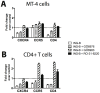
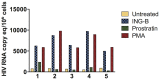
HIV infection is not cleared by antiretroviral drugs due to the presence of latently infected cells that are not eliminated with current therapies and persist in the blood and organs of infected patients. New compounds to activate these latent reservoirs have been evaluated so that, along with HAART, they can be used to activate latent virus and eliminate the latently infected cells resulting in eradication of viral infection. Here we describe three novel diterpenes isolated from the sap of Euphorbia tirucalli, a tropical shrub. These molecules, identified as ingenols, were modified at carbon 3 and termed ingenol synthetic derivatives (ISD). They activated the HIV-LTR in reporter cell lines and human PBMCs with latent virus in concentrations as low as 10 nM. ISDs were also able to inhibit the replication of HIV-1 subtype B and C in MT-4 cells and human PBMCs at concentrations of EC50 0.02 and 0.09 µM respectively, which are comparable to the EC50 of some antiretroviral currently used in AIDS treatment. Control of viral replication may be caused by downregulation of surface CD4, CCR5 and CXCR4 observed after ISD treatment in vitro. These compounds appear to be less cytotoxic than other diterpenes such as PMA and prostratin, with effective dose versus toxic dose TI>400. Although the mechanisms of action of the three ISDs are primarily attributed to the PKC pathway, downregulation of surface receptors and stimulation of the viral LTR might be differentially modulated by different PKC isoforms.
Conflict of interest statement
Figures

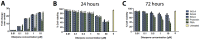

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

