Aging synaptic mitochondria exhibit dynamic proteomic changes while maintaining bioenergetic function
- PMID: 24827396
- PMCID: PMC4032798
- DOI: 10.18632/aging.100657
Aging synaptic mitochondria exhibit dynamic proteomic changes while maintaining bioenergetic function
Abstract
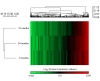
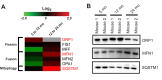
Aging correlates with a progressive impairment of mitochondrial homeostasis and is an influential factor for several forms of neurodegeneration. However, the mechanisms underlying age-related alterations in synaptosomal mitochondria, a neuronal mitochondria population highly susceptible to insults and critical for brain function, remain incompletely understood. Therefore this study investigates the synaptic mitochondrial proteomic and bioenergetic alterations that occur with age. The utilization of a state of the art quantitative proteomics approach allowed for the comparison of protein expression levels in synaptic mitochondria isolated from 5 (mature), 12 (old), and 24 (aged) month old mice. During the process of aging we find that dynamic proteomic alterations occur in synaptic mitochondria. Despite direct (mitochondrial DNA deletions) and indirect (increased antioxidant protein levels) signs of mitochondrial damage in the aged mice, there was an overall maintenance of mitochondrial function. Therefore the synaptic mitochondrial proteomic changes that occur with aging correlate with preservation of synaptic mitochondrial function.
Conflict of interest statement
The authors disclose that there are no actual or potential conflicts of interest.
Figures




References
-
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

