Development of EULAR recommendations for the reporting of clinical trial extension studies in rheumatology
- PMID: 24827533
- PMCID: PMC4431343
- DOI: 10.1136/annrheumdis-2013-204948
Development of EULAR recommendations for the reporting of clinical trial extension studies in rheumatology
Abstract
Objectives: Our initiative aimed to produce recommendations on post-randomised controlled trial (RCT) trial extension studies (TES) reporting using European League Against Rheumatism (EULAR) standard operating procedures in order to achieve more meaningful output and standardisation of reports.
Methods: We formed a task force of 22 participants comprising RCT experts, clinical epidemiologists and patient representatives. A two-stage Delphi survey was conducted to discuss the domains of evaluation of a TES and definitions. A '0-10' agreement scale assessed each domain and definition. The resulting set of recommendations was further refined and a final vote taken for task force acceptance.
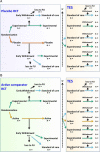
Results: Seven key domains and individual components were evaluated and led to agreed recommendations including definition of a TES (100% agreement), minimal data necessary (100% agreement), method of data analysis (agreement mean (SD) scores ranging between 7.9 (0.84) and 9.0 (2.16)) and reporting of results as well as ethical issues. Key recommendations included reporting of absolute numbers at each stage from the RCT to TES with reasons given for drop-out at each stage, and inclusion of a flowchart detailing change in numbers at each stage and focus (mean (SD) agreement 9.9 (0.36)). A final vote accepted the set of recommendations.
Conclusions: This EULAR task force provides recommendations for implementation in future TES to ensure a standardised approach to reporting. Use of this document should provide the rheumatology community with a more accurate and meaningful output from future TES, enabling better understanding and more confident application in clinical practice towards improving patient outcomes.
Keywords: DMARDs (biologic); Epidemiology; Rheumatoid Arthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- O'Dell JR, Leff R, Paulsen G, et al. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002;46:1164–70. - PubMed
-
- Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997;350:309–18. - PubMed
-
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9. - PubMed
-
- Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. - PubMed
-
- Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

