Mfsd2a is critical for the formation and function of the blood-brain barrier
- PMID: 24828040
- PMCID: PMC4134871
- DOI: 10.1038/nature13324
Mfsd2a is critical for the formation and function of the blood-brain barrier
Abstract
The central nervous system (CNS) requires a tightly controlled environment free of toxins and pathogens to provide the proper chemical composition for neural function. This environment is maintained by the 'blood-brain barrier' (BBB), which is composed of blood vessels whose endothelial cells display specialized tight junctions and extremely low rates of transcellular vesicular transport (transcytosis). In concert with pericytes and astrocytes, this unique brain endothelial physiological barrier seals the CNS and controls substance influx and efflux. Although BBB breakdown has recently been associated with initiation and perpetuation of various neurological disorders, an intact BBB is a major obstacle for drug delivery to the CNS. A limited understanding of the molecular mechanisms that control BBB formation has hindered our ability to manipulate the BBB in disease and therapy. Here we identify mechanisms governing the establishment of a functional BBB. First, using a novel tracer-injection method for embryos, we demonstrate spatiotemporal developmental profiles of BBB functionality and find that the mouse BBB becomes functional at embryonic day 15.5 (E15.5). We then screen for BBB-specific genes expressed during BBB formation, and find that major facilitator super family domain containing 2a (Mfsd2a) is selectively expressed in BBB-containing blood vessels in the CNS. Genetic ablation of Mfsd2a results in a leaky BBB from embryonic stages through to adulthood, but the normal patterning of vascular networks is maintained. Electron microscopy examination reveals a dramatic increase in CNS-endothelial-cell vesicular transcytosis in Mfsd2a(-/-) mice, without obvious tight-junction defects. Finally we show that Mfsd2a endothelial expression is regulated by pericytes to facilitate BBB integrity. These findings identify Mfsd2a as a key regulator of BBB function that may act by suppressing transcytosis in CNS endothelial cells. Furthermore, our findings may aid in efforts to develop therapeutic approaches for CNS drug delivery.
Figures
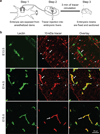
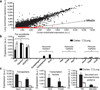
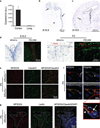

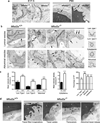
Comment in
-
Physiology: Double function at the blood-brain barrier.Nature. 2014 May 22;509(7501):432-3. doi: 10.1038/nature13339. Epub 2014 May 14. Nature. 2014. PMID: 24828036 No abstract available.
-
Blood-brain barrier: a dual life of MFSD2A?Neuron. 2014 May 21;82(4):728-30. doi: 10.1016/j.neuron.2014.05.012. Neuron. 2014. PMID: 24853933 Free PMC article.
References
-
- Armulik A, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

