The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR
- PMID: 24828042
- PMCID: PMC4263271
- DOI: 10.1038/nature13264
The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR
Abstract
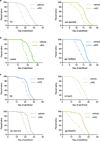
Metabolism and ageing are intimately linked. Compared with ad libitum feeding, dietary restriction consistently extends lifespan and delays age-related diseases in evolutionarily diverse organisms. Similar conditions of nutrient limitation and genetic or pharmacological perturbations of nutrient or energy metabolism also have longevity benefits. Recently, several metabolites have been identified that modulate ageing; however, the molecular mechanisms underlying this are largely undefined. Here we show that α-ketoglutarate (α-KG), a tricarboxylic acid cycle intermediate, extends the lifespan of adult Caenorhabditis elegans. ATP synthase subunit β is identified as a novel binding protein of α-KG using a small-molecule target identification strategy termed drug affinity responsive target stability (DARTS). The ATP synthase, also known as complex V of the mitochondrial electron transport chain, is the main cellular energy-generating machinery and is highly conserved throughout evolution. Although complete loss of mitochondrial function is detrimental, partial suppression of the electron transport chain has been shown to extend C. elegans lifespan. We show that α-KG inhibits ATP synthase and, similar to ATP synthase knockdown, inhibition by α-KG leads to reduced ATP content, decreased oxygen consumption, and increased autophagy in both C. elegans and mammalian cells. We provide evidence that the lifespan increase by α-KG requires ATP synthase subunit β and is dependent on target of rapamycin (TOR) downstream. Endogenous α-KG levels are increased on starvation and α-KG does not extend the lifespan of dietary-restricted animals, indicating that α-KG is a key metabolite that mediates longevity by dietary restriction. Our analyses uncover new molecular links between a common metabolite, a universal cellular energy generator and dietary restriction in the regulation of organismal lifespan, thus suggesting new strategies for the prevention and treatment of ageing and age-related diseases.
Figures






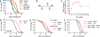



Comment in
-
Mitobolites: the elixir of life.Cell Metab. 2014 Jul 1;20(1):8-9. doi: 10.1016/j.cmet.2014.06.013. Cell Metab. 2014. PMID: 24988457 Free PMC article.
-
α-Ketoglutarate-A New Currency of Longevity.Sci Transl Med. 2014 Jul 9;6(244):244ec117. doi: 10.1126/scitranslmed.3009803. Sci Transl Med. 2014. PMID: 30008989 Free PMC article. No abstract available.
References
-
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

