An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice
- PMID: 24828079
- PMCID: PMC4269372
- DOI: 10.1126/scitranslmed.3008169
An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice
Abstract
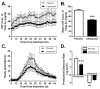
Serotonin signaling suppresses generation of amyloid-β (Aβ) in vitro and in animal models of Alzheimer's disease (AD). We show that in an aged transgenic AD mouse model (APP/PS1 plaque-bearing mice), the antidepressant citalopram, a selective serotonin reuptake inhibitor, decreased Aβ in brain interstitial fluid in a dose-dependent manner. Growth of individual amyloid plaques was assessed in plaque-bearing mice that were chronically administered citalopram. Citalopram arrested the growth of preexisting plaques and reduced the appearance of new plaques by 78%. In healthy human volunteers, citalopram's effects on Aβ production and Aβ concentrations in cerebrospinal fluid (CSF) were measured prospectively using stable isotope labeling kinetics, with CSF sampling during acute dosing of citalopram. Aβ production in CSF was slowed by 37% in the citalopram group compared to placebo. This change was associated with a 38% decrease in total CSF Aβ concentrations in the drug-treated group. The ability to safely decrease Aβ concentrations is potentially important as a preventive strategy for AD. This study demonstrates key target engagement for future AD prevention trials.
Copyright © 2014, American Association for the Advancement of Science.
Conflict of interest statement
Figures


Comment in
-
Comment on "An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice".Sci Transl Med. 2014 Dec 24;6(268):268le5. doi: 10.1126/scitranslmed.3010053. Sci Transl Med. 2014. PMID: 25540322 No abstract available.
-
Reply to comment on "An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice".Sci Transl Med. 2014 Dec 24;6(268):268lr4. doi: 10.1126/scitranslmed.3010609. Sci Transl Med. 2014. PMID: 25540323 Free PMC article.
References
-
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 Aug;60:1119. - PubMed
-
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999 Mar;45:358. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297:353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG041502/AG/NIA NIH HHS/United States
- R21 AG03969002/AG/NIA NIH HHS/United States
- K24 MH079510/MH/NIMH NIH HHS/United States
- R01 AG042513/AG/NIA NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- R01 NS067905/NS/NINDS NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- R24 GM136766/GM/NIGMS NIH HHS/United States
- R01 AG04150202/AG/NIA NIH HHS/United States
- R21 NS082529/NS/NINDS NIH HHS/United States
- R21 AG039690/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

