3-Aminoazetidin-2-one derivatives as N-acylethanolamine acid amidase (NAAA) inhibitors suitable for systemic administration
- PMID: 24828120
- PMCID: PMC4224963
- DOI: 10.1002/cmdc.201300546
3-Aminoazetidin-2-one derivatives as N-acylethanolamine acid amidase (NAAA) inhibitors suitable for systemic administration
Abstract
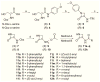
N-Acylethanolamine acid amidase (NAAA) is a cysteine hydrolase that catalyzes the hydrolysis of endogenous lipid mediators such as palmitoylethanolamide (PEA). PEA has been shown to exert anti-inflammatory and antinociceptive effects in animals by engaging peroxisome proliferator-activated receptor α (PPAR-α). Thus, preventing PEA degradation by inhibiting NAAA may provide a novel approach for the treatment of pain and inflammatory states. Recently, 3-aminooxetan-2-one compounds were identified as a class of highly potent NAAA inhibitors. The utility of these compounds is limited, however, by their low chemical and plasma stabilities. In the present study, we synthesized and tested a series of N-(2-oxoazetidin-3-yl)amides as a novel class of NAAA inhibitors with good potency and improved physicochemical properties, suitable for systemic administration. Moreover, we elucidated the main structural features of 3-aminoazetidin-2-one derivatives that are critical for NAAA inhibition.
Keywords: N-acylethanolamine acid amidase; cysteine hydrolase; inhibitors; structure-activity relationships; β-lactams.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

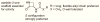




Similar articles
-
Design and synthesis of cyanamides as potent and selective N-acylethanolamine acid amidase inhibitors.Bioorg Med Chem. 2020 Jan 1;28(1):115195. doi: 10.1016/j.bmc.2019.115195. Epub 2019 Nov 11. Bioorg Med Chem. 2020. PMID: 31761726
-
Progress in the development of β-lactams as N-Acylethanolamine Acid Amidase (NAAA) inhibitors: Synthesis and SAR study of new, potent N-O-substituted derivatives.Eur J Med Chem. 2017 Jan 27;126:561-575. doi: 10.1016/j.ejmech.2016.11.039. Epub 2016 Nov 23. Eur J Med Chem. 2017. PMID: 27915171
-
Advances in the discovery of N-acylethanolamine acid amidase inhibitors.Pharmacol Res. 2014 Aug;86:11-7. doi: 10.1016/j.phrs.2014.04.011. Epub 2014 May 4. Pharmacol Res. 2014. PMID: 24798679 Free PMC article. Review.
-
Synthesis, structure-activity, and structure-stability relationships of 2-substituted-N-(4-oxo-3-oxetanyl) N-acylethanolamine acid amidase (NAAA) inhibitors.ChemMedChem. 2014 Feb;9(2):323-36. doi: 10.1002/cmdc.201300416. Epub 2014 Jan 8. ChemMedChem. 2014. PMID: 24403170
-
N-Acylethanolamine Acid Amidase (NAAA): Structure, Function, and Inhibition.J Med Chem. 2020 Jul 23;63(14):7475-7490. doi: 10.1021/acs.jmedchem.0c00191. Epub 2020 Mar 26. J Med Chem. 2020. PMID: 32191459 Review.
Cited by
-
Discovery and SAR Evolution of Pyrazole Azabicyclo[3.2.1]octane Sulfonamides as a Novel Class of Non-Covalent N-Acylethanolamine-Hydrolyzing Acid Amidase (NAAA) Inhibitors for Oral Administration.J Med Chem. 2021 Sep 23;64(18):13327-13355. doi: 10.1021/acs.jmedchem.1c00575. Epub 2021 Sep 1. J Med Chem. 2021. PMID: 34469137 Free PMC article.
-
A Potent Systemically Active N-Acylethanolamine Acid Amidase Inhibitor that Suppresses Inflammation and Human Macrophage Activation.ACS Chem Biol. 2015 Aug 21;10(8):1838-46. doi: 10.1021/acschembio.5b00114. Epub 2015 Apr 15. ACS Chem Biol. 2015. PMID: 25874594 Free PMC article.
-
LPS-induced modules of co-expressed genes in equine peripheral blood mononuclear cells.BMC Genomics. 2017 Jan 5;18(1):34. doi: 10.1186/s12864-016-3390-y. BMC Genomics. 2017. PMID: 28056766 Free PMC article.
-
A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries.Beilstein J Org Chem. 2021 May 18;17:1181-1312. doi: 10.3762/bjoc.17.90. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34136010 Free PMC article. Review.
-
Molecular mechanism of activation of the immunoregulatory amidase NAAA.Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):E10032-E10040. doi: 10.1073/pnas.1811759115. Epub 2018 Oct 9. Proc Natl Acad Sci U S A. 2018. PMID: 30301806 Free PMC article.
References
-
- Piomelli D, Giuffrida A, Calignano A, de Fonseca FR. Trends Pharmacol Sci. 2000;21:218–224. - PubMed
-
- Mazzari S, Canella R, Petrelli L, Marcolongo G, Leon A. Eur J Pharmacol. 1996;300:227–236. - PubMed
- Calignano A, La Rana G, Piomelli D. Eur J Pharmacol. 2001;419:191–198. - PubMed
- Darmani NA, Izzo AA, Degenhardt B, Valenti M, Scaglione G, Capasso R, Sorrentini I, Di Marzo V. Neuropharmacology. 2005;48:1154–1163. - PubMed
-
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. Mol Pharmacol. 2005;67:15–19. - PubMed
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. J Pharmacol Exp Ther. 2006;319:1051–1061. - PubMed
- Khasabova IA, Xiong Y, Coicou LG, Piomelli D, Seybold V. J Neurosci. 2012;32:12735–12743. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

