Lipid accumulation during the establishment of kleptoplasty in Elysia chlorotica
- PMID: 24828251
- PMCID: PMC4020867
- DOI: 10.1371/journal.pone.0097477
Lipid accumulation during the establishment of kleptoplasty in Elysia chlorotica
Abstract
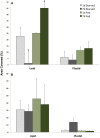
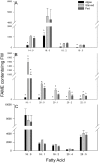
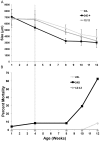
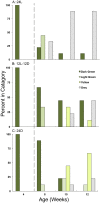
The establishment of kleptoplasty (retention of "stolen plastids") in the digestive tissue of the sacoglossan Elysia chlorotica Gould was investigated using transmission electron microscopy. Cellular processes occurring during the initial exposure to plastids were observed in laboratory raised animals ranging from 1-14 days post metamorphosis (dpm). These observations revealed an abundance of lipid droplets (LDs) correlating to plastid abundance. Starvation of animals resulted in LD and plastid decay in animals <5 dpm that had not yet achieved permanent kleptoplasty. Animals allowed to feed on algal prey (Vaucheria litorea C. Agardh) for 7 d or greater retained stable plastids resistant to cellular breakdown. Lipid analysis of algal and animal samples supports that these accumulating LDs may be of plastid origin, as the often algal-derived 20∶5 eicosapentaenoic acid was found in high abundance in the animal tissue. Subsequent culturing of animals in dark conditions revealed a reduced ability to establish permanent kleptoplasty in the absence of photosynthetic processes, coupled with increased mortality. Together, these data support an important role of photosynthetic lipid production in establishing and stabilizing this unique animal kleptoplasty.
Conflict of interest statement
Figures








Similar articles
-
Genome analysis of Elysia chlorotica Egg DNA provides no evidence for horizontal gene transfer into the germ line of this Kleptoplastic Mollusc.Mol Biol Evol. 2013 Aug;30(8):1843-52. doi: 10.1093/molbev/mst084. Epub 2013 May 2. Mol Biol Evol. 2013. PMID: 23645554 Free PMC article.
-
The making of a photosynthetic animal.J Exp Biol. 2011 Jan 15;214(Pt 2):303-11. doi: 10.1242/jeb.046540. J Exp Biol. 2011. PMID: 21177950 Free PMC article.
-
Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica.Proc Natl Acad Sci U S A. 2008 Nov 18;105(46):17867-71. doi: 10.1073/pnas.0804968105. Epub 2008 Nov 11. Proc Natl Acad Sci U S A. 2008. PMID: 19004808 Free PMC article.
-
Cell biology of the chloroplast symbiosis in sacoglossan sea slugs.Int Rev Cell Mol Biol. 2012;293:123-48. doi: 10.1016/B978-0-12-394304-0.00009-9. Int Rev Cell Mol Biol. 2012. PMID: 22251560 Review.
-
Crawling leaves: photosynthesis in sacoglossan sea slugs.J Exp Bot. 2013 Oct;64(13):3999-4009. doi: 10.1093/jxb/ert197. Epub 2013 Jul 11. J Exp Bot. 2013. PMID: 23846876 Review.
Cited by
-
On Being the Right Size as an Animal with Plastids.Front Plant Sci. 2017 Aug 17;8:1402. doi: 10.3389/fpls.2017.01402. eCollection 2017. Front Plant Sci. 2017. PMID: 28861094 Free PMC article. Review.
-
Functional kleptoplasts intermediate incorporation of carbon and nitrogen in cells of the Sacoglossa sea slug Elysia viridis.Sci Rep. 2020 Jun 29;10(1):10548. doi: 10.1038/s41598-020-66909-7. Sci Rep. 2020. PMID: 32601288 Free PMC article.
-
Aposymbiotic Specimen of the Photosynthetic Sea Slug Elysia crispata.Diversity (Basel). 2022 Apr 20;14(5):313. doi: 10.3390/d14050313. eCollection 2022 May. Diversity (Basel). 2022. PMID: 35911605 Free PMC article.
-
Inorganic carbon and nitrogen assimilation in cellular compartments of a benthic kleptoplastic foraminifer.Sci Rep. 2018 Jul 4;8(1):10140. doi: 10.1038/s41598-018-28455-1. Sci Rep. 2018. PMID: 29973634 Free PMC article.
-
Chloroplast digestion and the development of functional kleptoplasty in juvenile Elysia timida (Risso, 1818) as compared to short-term and non-chloroplast-retaining sacoglossan slugs.PLoS One. 2017 Oct 11;12(10):e0182910. doi: 10.1371/journal.pone.0182910. eCollection 2017. PLoS One. 2017. PMID: 29020043 Free PMC article.
References
-
- Trench RK (1975) Of “leaves that crawl”: functional chloroplasts in animal cells. Symp Soc Exp Biol: 229–265. - PubMed
-
- Rumpho ME, Dastoor F, Manhart J, Lee J (2006) The kleptoplast. In: Wise RR, Hoober JK, editors. The structure and function of plastids.New York: Advances in Photosynthesis and Respiration, Vol. 23 . pp. 451–473. doi:10.1007/978-1-4020-4061-0_23.
-
- Clark K, Jensen K, Stirts H (1990) Survey for functional kleptoplasty (chloroplast symbiosis) among West Atlantic Ascoglossa (Sacoglossa). Veliger 33: 339–345.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

