Dimeric pyrrole-imidazole alkaloids: synthetic approaches and biosynthetic hypotheses
- PMID: 24828265
- PMCID: PMC4096073
- DOI: 10.1039/c4cc02290d
Dimeric pyrrole-imidazole alkaloids: synthetic approaches and biosynthetic hypotheses
Abstract
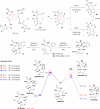
The pyrrole-imidazole alkaloids are a group of structurally unique and biologically interesting marine sponge metabolites. Among them, the cyclic dimers have caught synthetic chemists' attention particularly. Numerous synthetic strategies have been developed and various biosynthetic hypotheses have been proposed for these fascinating natural products. We discuss herein the synthetic approaches and the biosynthetic insights obtained from these studies.
Figures




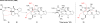
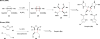



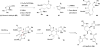
References
-
- Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311–335. - PMC - PubMed
- Newman DJ, Cragg GM, Snader KM. J. Nat. Prod. 2003;66:1022–1037. - PubMed
- Gerwick WH, Moore BS. Chem. Biol. 2012;19:85–98. - PMC - PubMed
- Cordell GA, Colvard MD. J. Nat. Prod. 2012;75:514–525. - PubMed
- Li JW-H, Vederas JC. Science. 2009;325:161–165. - PubMed
- Koehn FE, Carter GT. Nat. Rev. Drug Discovery. 2005;4:206–220. - PubMed
- MacCoss M, Baillie TA. Science. 2004;303:1810–1813. - PubMed
- Butler MS. J. Nat. Prod. 2004;67:2141–2153. - PubMed
- Breinbauer R, Vetter IR, Waldmann H. Angew. Chem. Int. Ed. 2002;41:2878–2890. - PubMed
-
- Cannon JS, Overman LE. Angew. Chem. Int. Ed. 2012;51:2–26. - PMC - PubMed
- Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc. Chem. Res. 2012;45:826–839. - PMC - PubMed
- Gutekunst WR, Baran PS. Chem. Soc. Rev. 2011;40:1976–1991. - PubMed
- Ishihara Y, Baran PS. Synlett. 2010:1733–1745.
- Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. Int. Ed. 2012;51:8960–9009. - PubMed
- Giri R, Shi B-F, Engle KM, Maugelc N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242–3272. - PubMed
- Que L, Jr., Tolman WB. Nature. 2008;455:333–340. - PubMed
- Clardy J, Walsh C. Nature. 2004;432:829–837. - PubMed
-
- Hoffmann H, Lindel T. Synthesis. 2003;35:1753–1783.
- Jacquot DEN, Lindel T. Curr. Org. Chem. 2005;9:1551–1565.
- Du H, He Y, Sivappa R, Lovely CJ. Synlett. 2006:965–992.
- Köck M, Grube A, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2007;46:6586–6594. - PubMed
- Weinreb SM. Nat. Prod. Rep. 2007;24:931–948. - PubMed
- Arndt H-D, Riedrich M. Angew. Chem. Int. Ed. 2008;47:4785–4788. - PubMed
- Wei Y, Zipse H. Eur. J. Org. Chem. 2008:3811–3816.
- Forte B, Malgesini B, Piutti C, Quartieri F, Scolaro A, Papeo G. Mar. Drugs. 2009;7:705–753. - PMC - PubMed
- Heasley B. Eur. J. Org. Chem. 2009:1477–1489.
- Shenvi RA, O'Malley DP, Baran PS. Acc. Chem. Res. 2009;42:530–541. - PMC - PubMed
- Al-Mourabit A, Zancanella MA, Tilvi S, Romo D. Nat. Prod. Rep. 2011;28:1229–1260. - PMC - PubMed
-
- Tan X, Chen C. Angew. Chem. Int. Ed. 2006;45:4345–4348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

