FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro
- PMID: 24828273
- PMCID: PMC4224080
- DOI: 10.1165/rcmb.2013-0363OC
FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro
Abstract
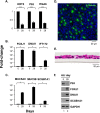
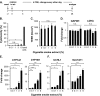
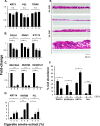
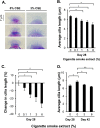
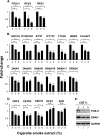
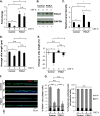
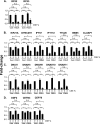
Airway epithelium ciliated cells play a central role in clearing the lung of inhaled pathogens and xenobiotics, and cilia length and coordinated beating are important for airway clearance. Based on in vivo studies showing that the airway epithelium of healthy smokers has shorter cilia than that of healthy nonsmokers, we investigated the mechanisms involved in cigarette smoke-mediated inhibition of ciliogenesis by assessing normal human airway basal cell differentiation in air-liquid interface (ALI) cultures in the presence of nontoxic concentrations of cigarette smoke extract (CSE). Measurements of cilia length from Day 28 ALI cultures demonstrated that CSE exposure was associated with shorter cilia (P < 0.05), reproducing the effect of cigarette smoking on cilia length observed in vivo. This phenotype correlated with a broad CSE-mediated suppression of genes involved in cilia-related transcriptional regulation, intraflagellar transport, cilia motility, structural integrity, and basal body development but not of control genes or epithelial barrier integrity. The CSE-mediated inhibition of cilia growth could be prevented by lentivirus-mediated overexpression of FOXJ1, the major cilia-related transcription factor, which led to partial reversal of expression of cilia-related genes suppressed by CSE. Together, the data suggest that components of cigarette smoke are responsible for a broad suppression of genes involved in cilia growth, but, by stimulating ciliogenesis with the transcription factor FOXJ1, it may be possible to maintain close to normal cilia length despite the stress of cigarette smoking.
Keywords: cigarette smoke; ciliogenesis.
Figures
Similar articles
-
RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium.Respir Res. 2013 Jul 3;14(1):70. doi: 10.1186/1465-9921-14-70. Respir Res. 2013. PMID: 23822649 Free PMC article.
-
Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2004 Apr;286(4):L650-7. doi: 10.1152/ajplung.00170.2003. Epub 2003 Jun 20. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 12818891
-
Cigarette smoke exposure impairs respiratory epithelial ciliogenesis.Am J Rhinol Allergy. 2009 Mar-Apr;23(2):117-22. doi: 10.2500/ajra.2009.23.3280. Am J Rhinol Allergy. 2009. PMID: 19401033
-
Transcriptional control of genes involved in ciliogenesis: a first step in making cilia.Biol Cell. 2010 Jul 9;102(9):499-513. doi: 10.1042/BC20100035. Biol Cell. 2010. PMID: 20690903 Review.
-
[Transcriptional control of ciliogenesis in animal development].Biol Aujourdhui. 2012;206(3):205-18. doi: 10.1051/jbio/2012023. Epub 2012 Nov 22. Biol Aujourdhui. 2012. PMID: 23171843 Review. French.
Cited by
-
A genome-wide association study of quantitative computed tomographic emphysema in Korean populations.Sci Rep. 2021 Aug 17;11(1):16692. doi: 10.1038/s41598-021-95887-7. Sci Rep. 2021. PMID: 34404834 Free PMC article.
-
Cilia and Mucociliary Clearance.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a028241. doi: 10.1101/cshperspect.a028241. Cold Spring Harb Perspect Biol. 2017. PMID: 27864314 Free PMC article. Review.
-
POU2AF1 Functions in the Human Airway Epithelium To Regulate Expression of Host Defense Genes.J Immunol. 2016 Apr 1;196(7):3159-67. doi: 10.4049/jimmunol.1502400. Epub 2016 Feb 29. J Immunol. 2016. PMID: 26927796 Free PMC article.
-
Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface.Sci Rep. 2015 Feb 2;5:8163. doi: 10.1038/srep08163. Sci Rep. 2015. PMID: 25641363 Free PMC article.
-
A lentiviral toolkit to monitor airway epithelial cell differentiation using bioluminescence.Am J Physiol Lung Cell Mol Physiol. 2024 Oct 1;327(4):L587-L599. doi: 10.1152/ajplung.00047.2024. Epub 2024 Aug 13. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39137525 Free PMC article.
References
-
- Lee RMKW, Forrest JB.Structure and function of ciliaCrystal RGeditor. The lung: scientific foundations2nd ed.. Philadelphia, PA: Lippincott-Raven Publishers; 1997459–478.
-
- Wanner A, Salathé M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. - PubMed
-
- Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol. 2007;35:116–129. - PubMed
-
- Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002;1:451–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

