Pharmacokinetics, electrophysiological, and morphological effects of the intravitreal injection of mycophenolic acid in rabbits
- PMID: 24828287
- PMCID: PMC4074757
- DOI: 10.1089/jop.2013.0236
Pharmacokinetics, electrophysiological, and morphological effects of the intravitreal injection of mycophenolic acid in rabbits
Abstract
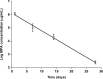
Purpose: To determine the half-life of mycophenolic acid (MPA) in the vitreous of New Zealand albino rabbits after intravitreal injection and the retinal toxicity of different doses of MPA.
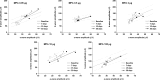
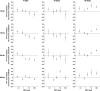
Methods: Ten micrograms of MPA (Roche Bioscience, Palo Alto, CA) was injected in the vitreous of 16 rabbits, animals were sacrificed at different time-points, and vitreous samples underwent high-performance liquid chromatography. For functional and morphological studies, 5 doses of MPA (0.05, 0.5, 2, 10, and 100 μg) were injected in the vitreous of 20 rabbits. As control, contralateral eyes were injected with aqueous vehicle. Electroretinograms (ERGs) were recorded before injection and at days 7, 15, and 30. Animals were sacrificed on day 30 and retinas were analyzed under light microscopy.
Results: MPA half-life in the vitreous was 5.0±0.3 days. ERG revealed photoreceptor functional impairment in eyes injected with 0.5 μg and higher on day 30, while eyes injected with 100 μg presented the same changes already from day 15. No morphological change was found.
Conclusions: MPA vitreous half-life is 5.0 days. Intravitreal injection of 0.5 μg MPA and higher causes dose- and time-related photoreceptor sensitivity decrease in rabbits. The MPA dose of 0.05 μg may be safe for intravitreal use in rabbits.
Figures



Similar articles
-
Evaluation of vitreous clearance and potential retinal toxicity of intravitreal lornoxicam (xefo).J Ocul Pharmacol Ther. 2013 Sep;29(7):627-32. doi: 10.1089/jop.2012.0194. Epub 2013 Apr 4. J Ocul Pharmacol Ther. 2013. PMID: 23556534
-
Ocular and systemic toxicity of intravitreal topotecan in rabbits for potential treatment of retinoblastoma.Exp Eye Res. 2013 Mar;108:103-9. doi: 10.1016/j.exer.2013.01.002. Epub 2013 Jan 16. Exp Eye Res. 2013. PMID: 23333535
-
Vitreous pharmacokinetics and electroretinographic findings after intravitreal injection of acyclovir in rabbits.Clinics (Sao Paulo). 2012 Aug;67(8):931-7. doi: 10.6061/clinics/2012(08)13. Clinics (Sao Paulo). 2012. PMID: 22948462 Free PMC article.
-
Ocular toxicity of intravitreal clarithromycin.Retina. 1999;19(5):442-6. doi: 10.1097/00006982-199909000-00013. Retina. 1999. PMID: 10546942
-
Safety of Intravitreal Injection of Stivant, a Biosimilar to Bevacizumab, in Rabbit Eyes.J Ophthalmic Vis Res. 2020 Aug 6;15(3):341-350. doi: 10.18502/jovr.v15i3.7453. eCollection 2020 Jul-Sep. J Ophthalmic Vis Res. 2020. PMID: 32864065 Free PMC article. Review.
Cited by
-
Corosolic acid: antiangiogenic activity and safety of intravitreal injection in rats eyes.Doc Ophthalmol. 2019 Jun;138(3):181-194. doi: 10.1007/s10633-019-09682-x. Epub 2019 Feb 26. Doc Ophthalmol. 2019. PMID: 30809742
-
Test-retest reliability of scotopic full-field electroretinograms in rabbits.Doc Ophthalmol. 2017 Jun;134(3):157-165. doi: 10.1007/s10633-017-9582-1. Epub 2017 Mar 16. Doc Ophthalmol. 2017. PMID: 28303363
-
Retinal Gene Therapy: Surgical Vector Delivery in the Translation to Clinical Trials.Front Neurosci. 2017 Apr 3;11:174. doi: 10.3389/fnins.2017.00174. eCollection 2017. Front Neurosci. 2017. PMID: 28420956 Free PMC article. Review.
-
Intravitreal injection of the synthetic peptide LyeTx I b, derived from a spider toxin, into the rabbit eye is safe and prevents neovascularization in a chorio-allantoic membrane model.J Venom Anim Toxins Incl Trop Dis. 2018 Nov 21;24:31. doi: 10.1186/s40409-018-0168-5. eCollection 2018. J Venom Anim Toxins Incl Trop Dis. 2018. PMID: 30479614 Free PMC article.
References
-
- Durrani K., Zakka F.R., Ahmed M., et al. . Systemic therapy with conventional and novel immunomodulatory agents for ocular inflammatory disease. Surv. Ophthalmol. 56:474–510, 2011 - PubMed
-
- LeHoang P.The gold standard of noninfectious uveitis: corticosteroids. Dev. Ophthalmol. 51:7–28, 2012 - PubMed
-
- Kruh J., and Foster C.S.Corticosteroid-sparing agents: conventional systemic immunosuppressants. Dev. Ophthalmol. 51:29–46, 2012 - PubMed
-
- Wiesner R.H., Steffen B.J., David K.M., et al. . Mycophenolate mofetil use is associated with decreased risk of late acute rejection in adult liver transplant recipients. Am. J. Transplant. 6:1609–1616, 2006 - PubMed
-
- Lake J.R., Shorr J.S., Steffen B.J., et al. . Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am. J. Transplant. 5:549–557, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

