Evolving marine biomimetics for regenerative dentistry
- PMID: 24828293
- PMCID: PMC4052322
- DOI: 10.3390/md12052877
Evolving marine biomimetics for regenerative dentistry
Abstract
New products that help make human tissue and organ regeneration more effective are in high demand and include materials, structures and substrates that drive cell-to-tissue transformations, orchestrate anatomical assembly and tissue integration with biology. Marine organisms are exemplary bioresources that have extensive possibilities in supporting and facilitating development of human tissue substitutes. Such organisms represent a deep and diverse reserve of materials, substrates and structures that can facilitate tissue reconstruction within lab-based cultures. The reason is that they possess sophisticated structures, architectures and biomaterial designs that are still difficult to replicate using synthetic processes, so far. These products offer tantalizing pre-made options that are versatile, adaptable and have many functions for current tissue engineers seeking fresh solutions to the deficiencies in existing dental biomaterials, which lack the intrinsic elements of biofunctioning, structural and mechanical design to regenerate anatomically correct dental tissues both in the culture dish and in vivo.
Figures




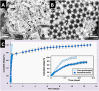
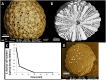



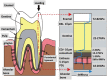
Similar articles
-
Tooth Bioengineering and Regenerative Dentistry.J Dent Res. 2019 Oct;98(11):1173-1182. doi: 10.1177/0022034519861903. J Dent Res. 2019. PMID: 31538866 Free PMC article.
-
Regenerative dentistry: translating advancements in basic science research to the dental practice.J Tenn Dent Assoc. 2010 Fall;90(4):12-8; quiz 18-9. J Tenn Dent Assoc. 2010. PMID: 21755797 Review.
-
Recent advancements in regenerative dentistry: A review.Mater Sci Eng C Mater Biol Appl. 2016 Dec 1;69:1383-90. doi: 10.1016/j.msec.2016.08.045. Epub 2016 Aug 20. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27612840 Review.
-
Diversification and enrichment of clinical biomaterials inspired by Darwinian evolution.Acta Biomater. 2016 Sep 15;42:33-45. doi: 10.1016/j.actbio.2016.06.039. Epub 2016 Jul 25. Acta Biomater. 2016. PMID: 27381524 Review.
-
Tooth bioengineering leads the next generation of dentistry.Int J Paediatr Dent. 2012 Nov;22(6):406-18. doi: 10.1111/j.1365-263X.2011.01206.x. Epub 2012 Jan 8. Int J Paediatr Dent. 2012. PMID: 22225846 Review.
Cited by
-
Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering.Materials (Basel). 2018 Sep 13;11(9):1702. doi: 10.3390/ma11091702. Materials (Basel). 2018. PMID: 30216991 Free PMC article. Review.
-
Scientific basis of dentistry.J Istanb Univ Fac Dent. 2017 Oct 2;51(3):64-71. doi: 10.17096/jiufd.04646. eCollection 2017. J Istanb Univ Fac Dent. 2017. PMID: 29114433 Free PMC article. Review.
-
Biomimetic Aspects of Oral and Dentofacial Regeneration.Biomimetics (Basel). 2020 Oct 12;5(4):51. doi: 10.3390/biomimetics5040051. Biomimetics (Basel). 2020. PMID: 33053903 Free PMC article. Review.
-
Immobilization of Titanium(IV) Oxide onto 3D Spongin Scaffolds of Marine Sponge Origin According to Extreme Biomimetics Principles for Removal of C.I. Basic Blue 9.Biomimetics (Basel). 2017 Mar 25;2(2):4. doi: 10.3390/biomimetics2020004. Biomimetics (Basel). 2017. PMID: 31105167 Free PMC article.
-
Marine Skeletons: Towards Hard Tissue Repair and Regeneration.Mar Drugs. 2018 Jul 2;16(7):225. doi: 10.3390/md16070225. Mar Drugs. 2018. PMID: 30004435 Free PMC article. Review.
References
-
- Vadgama P. Surfaces and Interfaces for Biomaterials. CRC Press, Woodhead Pubulisher Ltd.; Boca Raton, Cambridge, England: 2005. p. 802.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

