Pannexins form gap junctions with electrophysiological and pharmacological properties distinct from connexins
- PMID: 24828343
- PMCID: PMC4021813
- DOI: 10.1038/srep04955
Pannexins form gap junctions with electrophysiological and pharmacological properties distinct from connexins
Abstract
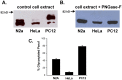
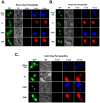
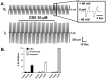
Stable expression of pannexin 1 (Panx1) and pannexin 3 (Panx3) resulted in functional gap junctions (GJs) in HeLa cells, but not in Neuro-2a (N2a) or PC-12 cells. The glycosylation pattern of expressed Panx1 varied greatly among different cell lines. In contrast to connexin (Cx) containing GJs (Cx-GJs), junctional conductance (Gj) of pannexin GJs (Panx-GJs) is very less sensitive to junctional voltage. Both Panx1 and Panx3 junctions favoured anionic dyes over cations to permeate. Though, carbenoxolone (CBX) and probenecid blocked Panx1 hemichannel activity, they had no effect on Panx1-GJs or Panx3-GJs. Extracellular loop 1 (E1) of Panx1 possibly bears the binding pocket. The Cx-GJ blocker heptanol blocked neither Panx1 hemichannel nor Panx-GJs. Unlike the GJs formed by most Cxs, CO2 did not uncouple Panx-GJs completely. Oxygen and glucose deprivation (OGD) caused lesser uncoupling of Panx-GJs compared to Cx43-GJs. These findings demonstrate properties of Panx-GJs that are distinctly different from Cx-GJs.
Figures



References
-
- Panchin Y. et al. A ubiquitous family of putative gap junction molecules. Curr Biol 10, R473–474 (2000). - PubMed
-
- Baranova A. et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83, 706–716 (2004). - PubMed
-
- Panchin Y. V. Evolution of gap junction proteins--the pannexin alternative. J Exp Biol 208, 1415–1419 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

