Functional characterization of two scFv-Fc antibodies from an HIV controller selected on soluble HIV-1 Env complexes: a neutralizing V3- and a trimer-specific gp41 antibody
- PMID: 24828352
- PMCID: PMC4020869
- DOI: 10.1371/journal.pone.0097478
Functional characterization of two scFv-Fc antibodies from an HIV controller selected on soluble HIV-1 Env complexes: a neutralizing V3- and a trimer-specific gp41 antibody
Erratum in
- PLoS One. 2014;9(8):e107089
Abstract
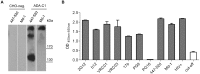
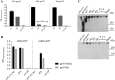
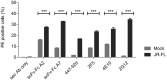
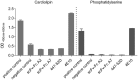
HIV neutralizing antibodies (nAbs) represent an important tool in view of prophylactic and therapeutic applications for HIV-1 infection. Patients chronically infected by HIV-1 represent a valuable source for nAbs. HIV controllers, including long-term non-progressors (LTNP) and elite controllers (EC), represent an interesting subgroup in this regard, as here nAbs can develop over time in a rather healthy immune system and in the absence of any therapeutic selection pressure. In this study, we characterized two particular antibodies that were selected as scFv antibody fragments from a phage immune library generated from an LTNP with HIV neutralizing antibodies in his plasma. The phage library was screened on recombinant soluble gp140 envelope (Env) proteins. Sequencing the selected peptide inserts revealed two major classes of antibody sequences. Binding analysis of the corresponding scFv-Fc derivatives to various trimeric and monomeric Env constructs as well as to peptide arrays showed that one class, represented by monoclonal antibody (mAb) A2, specifically recognizes an epitope localized in the pocket binding domain of the C heptad repeat (CHR) in the ectodomain of gp41, but only in the trimeric context. Thus, this antibody represents an interesting tool for trimer identification. MAb A7, representing the second class, binds to structural elements of the third variable loop V3 and neutralizes tier 1 and tier 2 HIV-1 isolates of different subtypes with matching critical amino acids in the linear epitope sequence. In conclusion, HIV controllers are a valuable source for the selection of functionally interesting antibodies that can be selected on soluble gp140 proteins with properties from the native envelope spike.
Conflict of interest statement
Figures


Similar articles
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
A Bispecific Antibody That Simultaneously Recognizes the V2- and V3-Glycan Epitopes of the HIV-1 Envelope Glycoprotein Is Broader and More Potent than Its Parental Antibodies.mBio. 2020 Jan 14;11(1):e03080-19. doi: 10.1128/mBio.03080-19. mBio. 2020. PMID: 31937648 Free PMC article.
-
A Highly Unusual V1 Region of Env in an Elite Controller of HIV Infection.J Virol. 2019 May 1;93(10):e00094-19. doi: 10.1128/JVI.00094-19. Print 2019 May 15. J Virol. 2019. PMID: 30842322 Free PMC article.
-
Structure-based vaccine design in HIV: blind men and the elephant?Curr Pharm Des. 2010;16(33):3744-53. doi: 10.2174/138161210794079173. Curr Pharm Des. 2010. PMID: 21128885 Free PMC article. Review.
-
Phages and HIV-1: from display to interplay.Int J Mol Sci. 2012;13(4):4727-4794. doi: 10.3390/ijms13044727. Epub 2012 Apr 13. Int J Mol Sci. 2012. PMID: 22606007 Free PMC article. Review.
Cited by
-
A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations.Cell Rep. 2021 Jul 27;36(4):109433. doi: 10.1016/j.celrep.2021.109433. Epub 2021 Jul 7. Cell Rep. 2021. PMID: 34273271 Free PMC article.
-
Hybridoma technology: is it still useful?Curr Res Immunol. 2021 Mar 22;2:32-40. doi: 10.1016/j.crimmu.2021.03.002. eCollection 2021. Curr Res Immunol. 2021. PMID: 35492397 Free PMC article. Review.
-
Construction of Human Immune and Naive scFv Phage Display Libraries.Methods Mol Biol. 2023;2702:15-37. doi: 10.1007/978-1-0716-3381-6_2. Methods Mol Biol. 2023. PMID: 37679613
-
Optimization of the EC26-2A4 Epitope in the gp41 Membrane Proximal External Region Targeted by Neutralizing Antibodies from an Elite Controller.AIDS Res Hum Retroviruses. 2018 Apr;34(4):365-374. doi: 10.1089/AID.2017.0250. Epub 2018 Jan 25. AIDS Res Hum Retroviruses. 2018. PMID: 29262692 Free PMC article.
-
Generation of Recombinant Antibodies Against Toxins and Viruses by Phage Display for Diagnostics and Therapy.Adv Exp Med Biol. 2016;917:55-76. doi: 10.1007/978-3-319-32805-8_4. Adv Exp Med Biol. 2016. PMID: 27236552 Free PMC article. Review.
References
-
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, et al. (2000) Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 6: 200–206. - PubMed
-
- Ferrantelli F, Hofmann-Lehmann R, Rasmussen RA, Wang T, Xu W, et al. (2003) Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS 17: 301–309. - PubMed
-
- Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, et al. (2010) Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol 84: 1302–1313. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

