Distribution of peripheral PrP(Sc) in sheep with naturally acquired scrapie
- PMID: 24828439
- PMCID: PMC4020850
- DOI: 10.1371/journal.pone.0097768
Distribution of peripheral PrP(Sc) in sheep with naturally acquired scrapie
Abstract
Accumulation of prion protein (PrPSc) in the central nervous system is the hallmark of transmissible spongiform encephalopathies. However, in some of these diseases such as scrapie or chronic wasting disease, the PrPSc can also accumulate in other tissues, particularly in the lymphoreticular system. In recent years, PrPSc in organs other than nervous and lymphoid have been described, suggesting that distribution of this protein in affected individuals may be much larger than previously thought. In the present study, 11 non-nervous/non-lymphoid organs from 16 naturally scrapie infected sheep in advanced stages of the disease were examined for the presence of PrPSc. Fourteen infected sheep were of the ARQ/ARQ PRNP genotype and 2 of the VRQ/VRQ, where the letters A, R, Q, and V represent the codes for amino-acids alanine, arginine, glutamine and valine, respectively. Adrenal gland, pancreas, heart, skin, urinary bladder and mammary gland were positive for PrPSc by immunohistochemistry and IDEXX HerdChek scrapie/BSE Antigen EIA Test in at least one animal. Lung, liver, kidney and skeletal muscle exhibited PrPSc deposits by immunohistochemistry only. To our knowledge, this is the first report regarding the presence of PrPSc in the heart, pancreas and urinary bladder in naturally acquired scrapie infections. In some other organs examined, in which PrPSc had been previously detected, PrPSc immunolabeling was observed to be associated with new structures within those organs. The results of the present study illustrate a wide dissemination of PrPSc in both ARQ/ARQ and VRQ/VRQ infected sheep, even when the involvement of the lymphoreticular system is scarce or absent, thus highlighting the role of the peripheral nervous system in the spread of PrPSc.
Conflict of interest statement
Figures
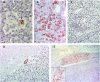




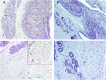
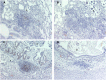
References
-
- Beekes M, McBride PA (2007) The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J 274: 588–605. - PubMed
-
- Jeffrey M, Gonzalez L (2004) Pathology and pathogenesis of bovine spongiform encephalopathy and scrapie. Curr Top Microbiol Immunol 284: 65–97. - PubMed
-
- van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA (1999) Scrapie-associated prion protein in the gastrointestinal tract of sheep with natural scrapie. J Comp Pathol 121: 55–63. - PubMed
-
- Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, et al. (2000) Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol 81: 3115–3126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

