Thymosin β4 up-regulation of microRNA-146a promotes oligodendrocyte differentiation and suppression of the Toll-like proinflammatory pathway
- PMID: 24828499
- PMCID: PMC4094061
- DOI: 10.1074/jbc.M113.529966
Thymosin β4 up-regulation of microRNA-146a promotes oligodendrocyte differentiation and suppression of the Toll-like proinflammatory pathway
Abstract
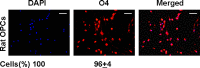
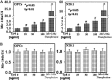
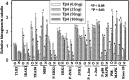
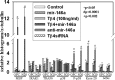
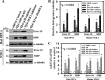
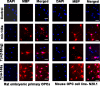
Thymosin β4 (Tβ4), a G-actin-sequestering peptide, improves neurological outcome in rat models of neurological injury. Tissue inflammation results from neurological injury, and regulation of the inflammatory response is vital for neurological recovery. The innate immune response system, which includes the Toll-like receptor (TLR) proinflammatory signaling pathway, regulates tissue injury. We hypothesized that Tβ4 regulates the TLR proinflammatory signaling pathway. Because oligodendrogenesis plays an important role in neurological recovery, we employed an in vitro primary rat embryonic cell model of oligodendrocyte progenitor cells (OPCs) and a mouse N20.1 OPC cell line to measure the effects of Tβ4 on the TLR pathway. Cells were grown in the presence of Tβ4, ranging from 25 to 100 ng/ml (RegeneRx Biopharmaceuticals Inc., Rockville, MD), for 4 days. Quantitative real-time PCR data demonstrated that Tβ4 treatment increased expression of microRNA-146a (miR-146a), a negative regulator the TLR signaling pathway, in these two cell models. Western blot analysis showed that Tβ4 treatment suppressed expression of IL-1 receptor-associated kinase 1 (IRAK1) and tumor necrosis factor receptor-associated factor 6 (TRAF6), two proinflammatory cytokines of the TLR signaling pathway. Transfection of miR-146a into both primary rat embryonic OPCs and mouse N20.1 OPCs treated with Tβ4 demonstrated an amplification of myelin basic protein (MBP) expression and differentiation of OPC into mature MBP-expressing oligodendrocytes. Transfection of anti-miR-146a nucleotides reversed the inhibitory effect of Tβ4 on IRAK1 and TRAF6 and decreased expression of MBP. These data suggest that Tβ4 suppresses the TLR proinflammatory pathway by up-regulating miR-146a.
Keywords: Actin; MicroRNA (miRNA); Myelin; Oligodendrocyte; Toll-like Receptor (TLR).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








Similar articles
-
A new insight into thymosin β4, a promising therapeutic approach for neurodegenerative disorders.J Cell Physiol. 2020 Apr;235(4):3270-3279. doi: 10.1002/jcp.29293. Epub 2019 Oct 14. J Cell Physiol. 2020. PMID: 31612500 Review.
-
Thymosin beta4 promotes oligodendrogenesis in the demyelinating central nervous system.Neurobiol Dis. 2016 Apr;88:85-95. doi: 10.1016/j.nbd.2016.01.010. Epub 2016 Jan 12. Neurobiol Dis. 2016. PMID: 26805386
-
Thymosin β 4 mediates oligodendrocyte differentiation by upregulating p38 MAPK.Glia. 2012 Dec;60(12):1826-38. doi: 10.1002/glia.22400. Epub 2012 Aug 1. Glia. 2012. PMID: 23073962 Free PMC article.
-
miR-146a ameliorates liver ischemia/reperfusion injury by suppressing IRAK1 and TRAF6.PLoS One. 2014 Jul 2;9(7):e101530. doi: 10.1371/journal.pone.0101530. eCollection 2014. PLoS One. 2014. PMID: 24987958 Free PMC article.
-
MicroRNA in TLR signaling and endotoxin tolerance.Cell Mol Immunol. 2011 Sep;8(5):388-403. doi: 10.1038/cmi.2011.26. Epub 2011 Aug 8. Cell Mol Immunol. 2011. PMID: 21822296 Free PMC article. Review.
Cited by
-
The therapeutic potential of microRNAs to ameliorate spinal cord injury by regulating oligodendrocyte progenitor cells and remyelination.Front Cell Neurosci. 2024 May 15;18:1404463. doi: 10.3389/fncel.2024.1404463. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38812792 Free PMC article. Review.
-
Ectopic Expression of Human Thymosin β4 Confers Resistance to Legionella pneumophila during Pulmonary and Systemic Infection in Mice.Infect Immun. 2021 Mar 17;89(4):e00735-20. doi: 10.1128/IAI.00735-20. Print 2021 Mar 17. Infect Immun. 2021. PMID: 33468581 Free PMC article.
-
Serum Thymosin β4 Concentrations in Obstructive Sleep Apnea Syndrome Patients.J Clin Lab Anal. 2016 Sep;30(5):736-40. doi: 10.1002/jcla.21930. Epub 2016 Apr 18. J Clin Lab Anal. 2016. PMID: 27086675 Free PMC article.
-
miR-146a mediates thymosin β4 induced neurovascular remodeling of diabetic peripheral neuropathy in type-II diabetic mice.Brain Res. 2019 Mar 15;1707:198-207. doi: 10.1016/j.brainres.2018.11.039. Epub 2018 Nov 27. Brain Res. 2019. PMID: 30500399 Free PMC article.
-
Effect and mechanism of miR-146a on malignant biological behaviors of lung adenocarcinoma cell line.Oncol Lett. 2020 Jun;19(6):3643-3652. doi: 10.3892/ol.2020.11474. Epub 2020 Mar 23. Oncol Lett. 2020. PMID: 32382320 Free PMC article.
References
-
- Huff T., Müller C. S., Otto A. M., Netzker R., Hannappel E. (2001) β-Thymosins, small acidic peptides with multiple functions. Int. J. Biochem. Cell Biol. 33, 205–220 - PubMed
-
- Goldstein A. L., Hannappel E., Kleinman H. K. (2005) Thymosin β4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol. Med. 11, 421–429 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

