Allosteric regulation of the human and mouse deoxyribonucleotide triphosphohydrolase sterile α-motif/histidine-aspartate domain-containing protein 1 (SAMHD1)
- PMID: 24828500
- PMCID: PMC4140260
- DOI: 10.1074/jbc.M114.571091
Allosteric regulation of the human and mouse deoxyribonucleotide triphosphohydrolase sterile α-motif/histidine-aspartate domain-containing protein 1 (SAMHD1)
Abstract
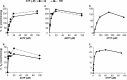
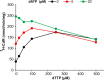
The deoxyribonucleotide triphosphohydrolase SAMHD1 restricts lentiviral infection by depleting the dNTPs required for viral DNA synthesis. In cultured human fibroblasts SAMHD1 is expressed maximally during quiescence preventing accumulation of dNTPs outside S phase. siRNA silencing of SAMHD1 increases dNTP pools, stops cycling human cells in G1, and blocks DNA replication. Surprisingly, knock-out of the mouse gene does not affect the well being of the animals. dNTPs are both substrates and allosteric effectors for SAMHD1. In the crystal structure each subunit of the homotetrameric protein contains one substrate-binding site and two nonidentical effector-binding sites, site 1 binding dGTP, site 2 dGTP or dATP. Here we compare allosteric properties of pure recombinant human and mouse SAMHD1. Both enzymes are activated 3-4-fold by allosteric effectors. We propose that in quiescent cells where SAMHD1 is maximally expressed GTP binds to site 1 with very high affinity, stabilizing site 2 of the tetrameric structure. Any canonical dNTP can bind to site 2 and activate SAMHD1, but in cells only dATP or dTTP are present at sufficient concentrations. The apparent Km for dATP at site 2 is ∼10 μm for mouse and 1 μm for human SAMHD1, for dTTP the corresponding values are 50 and 2 μm. Tetrameric SAMHD1 is activated for the hydrolysis of any dNTP only after binding of a dNTP to site 2. The lower Km constants for human SAMHD1 induce activation at lower cellular concentrations of dNTPs thereby limiting the size of dNTP pools more efficiently in quiescent human cells.
Keywords: Allosteric Regulation; DNA Enzyme; DNA Replication; Enzyme Kinetics; Enzyme Mechanism.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Goldstone D. C., Ennis-Adeniran V., Hedden J. J., Groom H. C., Rice G. I., Christodoulou E., Walker P. A., Kelly G., Haire L. F., Yap M. W., de Carvalho L. P., Stoye J. P., Crow Y. J., Taylor I. A., Webb M. (2011) HIV-1 restriction factor SAMHD1 is a deoxyribonucleoside triphosphate triphosphohydrolase. Nature 480, 379–382 - PubMed
-
- Rice G. I., Bond J., Asipu A., Brunette R. L., Manfield I. W., Carr I. M., Fuller J. C., Jackson R. M., Lamb T., Briggs T. A., Ali M., Gornall H., Couthard L. R., Aeby A., Attard-Montalto S. P., Bertini E., Bodemer C., Brockmann K., Brueton L. A., Corry P. C., Desguerre I., Fazzi E., Cazorla A. G., Gener B., Hamel B. C., Heiberg A., Hunter M., van der Knaap M. S., Kumar R., Lagae L., Landrieu P. G., Lourenco C. M., Marom D., McDermott M. F., van der Merwe W., Orcesi S., Prendiville J. S., Rasmussen M., Shalev S. A., Soler D. M., Shinawi M., Spiegel R., Tan T. Y., Vanderver A., Wakeling E. L., Wassmer E., Whittaker E., Lebon P., Stetson D. B., Bonthron D. T., Crow Y. J. (2009) Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

