Protein kinase D1 is essential for Ras-induced senescence and tumor suppression by regulating senescence-associated inflammation
- PMID: 24828530
- PMCID: PMC4040603
- DOI: 10.1073/pnas.1310972111
Protein kinase D1 is essential for Ras-induced senescence and tumor suppression by regulating senescence-associated inflammation
Abstract
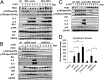
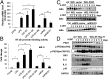
Oncogene-induced senescence (OIS) is an initial barrier to tumor development. Reactive oxygen species (ROS) is critical for oncogenic Ras OIS, but the downstream effectors to mediate ROS signaling are still relatively elusive. Senescent cells develop a senescence-associated secretory phenotype (SASP). However, the mechanisms underlying the regulation of the SASP are largely unknown. Here, we identify protein kinase D1 (PKD1) as a downstream effector of ROS signaling to mediate Ras OIS and SASP. PKD1 is activated by oncogenic Ras expression and PKD1 promotes Ras OIS by mediating inflammatory cytokines interleukin-6 (IL-6) and interleukin-8 (IL-8) via modulation of NF-κB activity. We demonstrate that ROS-protein kinase Cδ (PKCδ)-PKD1 axis is essential for the establishment and maintenance of IL-6/IL8 induction. In addition, ablation of PKD1 causes the bypass of Ras OIS, and promotes cell transformation and tumorigenesis. Together, these findings uncover a previously unidentified role of ROS-PKCδ-PKD1 pathway in Ras OIS and SASP regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. - PubMed
-
- Lee AC, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274(12):7936–7940. - PubMed
-
- Wu C, Miloslavskaya I, Demontis S, Maestro R, Galaktionov K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature. 2004;432(7017):640–645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

