Defective transport of the obesity mutant PC1/3 N222D contributes to loss of function
- PMID: 24828610
- PMCID: PMC4060179
- DOI: 10.1210/en.2013-1985
Defective transport of the obesity mutant PC1/3 N222D contributes to loss of function
Abstract
Mutations in the PCSK1 gene encoding prohormone convertase 1/3 (PC1/3) are strongly associated with obesity in humans. The PC1/3(N222D) mutant mouse thus far represents the only mouse model that mimics the PC1/3 obesity phenotype in humans. The present investigation addresses the cell biology of the N222D mutation. Metabolic labeling experiments reveal a clear defect in the kinetics of insulin biosynthesis in islets from PC1/3(N222D) mutant mice, resulting in an increase in both proinsulin and its processing intermediates, predominantly lacking cleavage at the Arg-Arg site. Although the mutant PC1/3 zymogen is correctly processed to the 87-kDa form, pulse-chase immunoprecipitation experiments, labeling, and immunohistochemical experiments using uncleavable variants all demonstrate that the PC1/3-N222D protein is largely mislocalized compared with similar wild-type (WT) constructs, being predominantly retained in the endoplasmic reticulum. The PC1/3-N222D mutant also undergoes more efficient degradation via the ubiquitin-proteasome system than the WT enzyme. Lastly, the mutant PC1/3-N222D protein coimmunoprecipitates with WT PC1/3 and exerts a modest effect on intracellular retention of the WT enzyme. These profound alterations in the cell biology of PC1/3-N222D are likely to contribute to the defective insulin biosynthetic events observed in the mutant mice and may be relevant to the dramatic contributions of polymorphisms in this gene to human obesity.
Figures
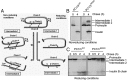
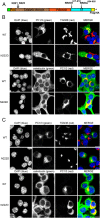
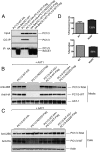
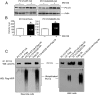
Comment in
-
The genetics of obesity meets basic cell biology through prohormone convertase 1/3.Endocrinology. 2014 Jul;155(7):2343-5. doi: 10.1210/en.2014-1376. Endocrinology. 2014. PMID: 24950989 No abstract available.
References
-
- Steiner DF. On the discovery of precursor processing. Methods Mol Biol. 2011;768:3–11 - PubMed
-
- Zhou Y, Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem. 1994;269:18408–18413 - PubMed
-
- Milgram SL, Mains RE. Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J Cell Sci. 1994;107:737–745 - PubMed
-
- Benjannet S, Rondeau N, Paquet L, et al. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993;294(pt 3):735–743 - PMC - PubMed
-
- Coates LC, Birch NP. Posttranslational maturation of the prohormone convertase sPC3 in vitro. J Neurochem. 1997;68:828–836 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

