Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system
- PMID: 24828639
- PMCID: PMC4019799
- DOI: 10.1523/JNEUROSCI.4993-13.2014
Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system
Abstract
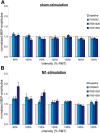
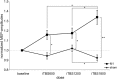
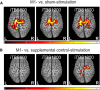
Theta burst stimulation (TBS), a specific protocol of repetitive transcranial magnetic stimulation (rTMS), induces changes in cortical excitability that last beyond stimulation. TBS-induced aftereffects, however, vary between subjects, and the mechanisms underlying these aftereffects to date remain poorly understood. Therefore, the purpose of this study was to investigate whether increasing the number of pulses of intermittent TBS (iTBS) (1) increases cortical excitability as measured by motor-evoked potentials (MEPs) and (2) alters functional connectivity measured using resting-state fMRI, in a dose-dependent manner. Sixteen healthy, human subjects received three serially applied iTBS blocks of 600 pulses over the primary motor cortex (M1 stimulation) and the parieto-occipital vertex (sham stimulation) to test for dose-dependent iTBS effects on cortical excitability and functional connectivity (four sessions in total). iTBS over M1 increased MEP amplitudes compared with sham stimulation after each stimulation block. Although the increase in MEP amplitudes did not differ between the first and second block of M1 stimulation, we observed a significant increase after three blocks (1800 pulses). Furthermore, iTBS enhanced resting-state functional connectivity between the stimulated M1 and premotor regions in both hemispheres. Functional connectivity between M1 and ipsilateral dorsal premotor cortex further increased dose-dependently after 1800 pulses of iTBS over M1. However, no correlation between changes in MEP amplitudes and functional connectivity was detected. In summary, our data show that increasing the number of iTBS stimulation blocks results in dose-dependent effects at the local level (cortical excitability) as well as at a systems level (functional connectivity) with a dose-dependent enhancement of dorsal premotor cortex-M1 connectivity.
Keywords: functional connectivity; iTBS; neural plasticity; premotor cortex; resting-state fMRI; supplementary motor area.
Copyright © 2014 the authors 0270-6474/14/346849-11$15.00/0.
Figures



Similar articles
-
Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS.Neuroimage. 2015 Sep;118:209-18. doi: 10.1016/j.neuroimage.2015.06.004. Epub 2015 Jun 5. Neuroimage. 2015. PMID: 26052083 Free PMC article.
-
Modulation of I-wave generating pathways by theta-burst stimulation: a model of plasticity induction.J Physiol. 2019 Dec;597(24):5963-5971. doi: 10.1113/JP278636. Epub 2019 Nov 12. J Physiol. 2019. PMID: 31647123
-
Interindividual differences in motor network connectivity and behavioral response to iTBS in stroke patients.Neuroimage Clin. 2017 Jun 4;15:559-571. doi: 10.1016/j.nicl.2017.06.006. eCollection 2017. Neuroimage Clin. 2017. PMID: 28652969 Free PMC article.
-
Efficacy and Time Course of Theta Burst Stimulation in Healthy Humans.Brain Stimul. 2015 Jul-Aug;8(4):685-92. doi: 10.1016/j.brs.2015.03.004. Epub 2015 Mar 26. Brain Stimul. 2015. PMID: 26014214 Review.
-
A systematic review of the neurobiological effects of theta-burst stimulation (TBS) as measured using functional magnetic resonance imaging (fMRI).Brain Struct Funct. 2023 May;228(3-4):717-749. doi: 10.1007/s00429-023-02634-x. Epub 2023 Apr 19. Brain Struct Funct. 2023. PMID: 37072625 Free PMC article.
Cited by
-
Functional connectivity drives stroke recovery: shifting the paradigm from correlation to causation.Brain. 2022 May 24;145(4):1211-1228. doi: 10.1093/brain/awab469. Brain. 2022. PMID: 34932786 Free PMC article. Review.
-
Efficacy and Safety of High-Dose TBS on Poststroke Upper Extremity Motor Impairment: A Randomized Controlled Trial.Stroke. 2024 Sep;55(9):2212-2220. doi: 10.1161/STROKEAHA.124.046597. Epub 2024 Jul 17. Stroke. 2024. PMID: 39016009 Free PMC article. Clinical Trial.
-
Developing a reverse translational model of low-intensity rTMS in alcohol use disorder: The influence of theta burst stimulation protocols on binge alcohol drinking in mice.Transcranial Magn Stimul. 2025 Aug;4:100098. doi: 10.1016/j.transm.2025.100098. Epub 2025 Apr 18. Transcranial Magn Stimul. 2025. PMID: 40585525 Free PMC article.
-
Augmented efficacy of intermittent theta burst stimulation on the virtual reality-based cycling training for upper limb function in patients with stroke: a double-blinded, randomized controlled trial.J Neuroeng Rehabil. 2021 May 31;18(1):91. doi: 10.1186/s12984-021-00885-5. J Neuroeng Rehabil. 2021. PMID: 34059090 Free PMC article. Clinical Trial.
-
Intermittent Theta-Burst Transcranial Magnetic Stimulation Alters Electrical Properties of Fast-Spiking Neocortical Interneurons in an Age-Dependent Fashion.Front Neural Circuits. 2016 Mar 30;10:22. doi: 10.3389/fncir.2016.00022. eCollection 2016. Front Neural Circuits. 2016. PMID: 27065812 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
