Feature interactions enable decoding of sensorimotor transformations for goal-directed movement
- PMID: 24828640
- PMCID: PMC4099499
- DOI: 10.1523/JNEUROSCI.5173-13.2014
Feature interactions enable decoding of sensorimotor transformations for goal-directed movement
Abstract
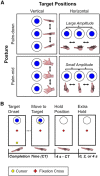
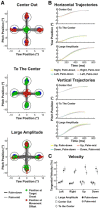
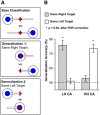
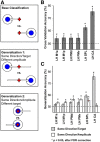
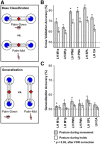
Neurophysiology and neuroimaging evidence shows that the brain represents multiple environmental and body-related features to compute transformations from sensory input to motor output. However, it is unclear how these features interact during goal-directed movement. To investigate this issue, we examined the representations of sensory and motor features of human hand movements within the left-hemisphere motor network. In a rapid event-related fMRI design, we measured cortical activity as participants performed right-handed movements at the wrist, with either of two postures and two amplitudes, to move a cursor to targets at different locations. Using a multivoxel analysis technique with rigorous generalization tests, we reliably distinguished representations of task-related features (primarily target location, movement direction, and posture) in multiple regions. In particular, we identified an interaction between target location and movement direction in the superior parietal lobule, which may underlie a transformation from the location of the target in space to a movement vector. In addition, we found an influence of posture on primary motor, premotor, and parietal regions. Together, these results reveal the complex interactions between different sensory and motor features that drive the computation of sensorimotor transformations.
Keywords: MVPA; fMRI; sensorimotor transformations.
Copyright © 2014 the authors 0270-6474/14/346860-14$15.00/0.
Figures



References
-
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001;29:919–1188.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
