Microstimulation of the human substantia nigra alters reinforcement learning
- PMID: 24828643
- PMCID: PMC4019802
- DOI: 10.1523/JNEUROSCI.5445-13.2014
Microstimulation of the human substantia nigra alters reinforcement learning
Abstract
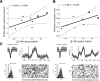
Animal studies have shown that substantia nigra (SN) dopaminergic (DA) neurons strengthen action-reward associations during reinforcement learning, but their role in human learning is not known. Here, we applied microstimulation in the SN of 11 patients undergoing deep brain stimulation surgery for the treatment of Parkinson's disease as they performed a two-alternative probability learning task in which rewards were contingent on stimuli, rather than actions. Subjects demonstrated decreased learning from reward trials that were accompanied by phasic SN microstimulation compared with reward trials without stimulation. Subjects who showed large decreases in learning also showed an increased bias toward repeating actions after stimulation trials; therefore, stimulation may have decreased learning by strengthening action-reward associations rather than stimulus-reward associations. Our findings build on previous studies implicating SN DA neurons in preferentially strengthening action-reward associations during reinforcement learning.
Keywords: Parkinson's disease; dopamine; human; microstimulation; reinforcement learning; substantia nigra.
Copyright © 2014 the authors 0270-6474/14/346887-09$15.00/0.
Figures




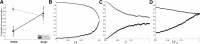
Comment in
-
A role for the human substantia nigra in reinforcement learning.J Neurosci. 2014 Sep 24;34(39):12947-9. doi: 10.1523/JNEUROSCI.2854-14.2014. J Neurosci. 2014. PMID: 25253842 Free PMC article. No abstract available.
References
-
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
