Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning
- PMID: 24828650
- PMCID: PMC4019806
- DOI: 10.1523/JNEUROSCI.3366-13.2014
Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning
Abstract
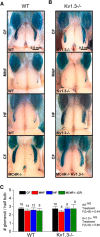
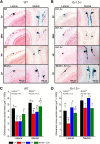
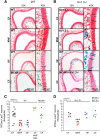
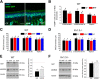
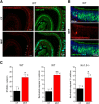
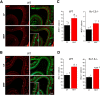
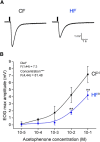
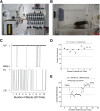
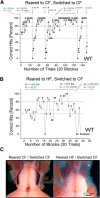
Currently, 65% of Americans are overweight, which leads to well-supported cardiovascular and cognitive declines. Little, however, is known concerning obesity's impact on sensory systems. Because olfaction is linked with ingestive behavior to guide food choice, its potential dysfunction during obesity could evoke a positive feedback loop to perpetuate poor ingestive behaviors. To determine the effect of chronic energy imbalance and reveal any structural or functional changes associated with obesity, we induced long-term, diet-induced obesity by challenging mice to high-fat diets: (1) in an obesity-prone (C57BL/6J) and obesity-resistant (Kv1.3(-/-)) line of mice, and compared this with (2) late-onset, genetic-induced obesity in MC4R(-/-) mice in which diabetes secondarily precipitates after disruption of the hypothalamic axis. We report marked loss of olfactory sensory neurons and their axonal projections after exposure to a fatty diet, with a concomitant reduction in electro-olfactogram amplitude. Loss of olfactory neurons and associated circuitry is linked to changes in neuronal proliferation and normal apoptotic cycles. Using a computer-controlled, liquid-based olfactometer, mice maintained on fatty diets learn reward-reinforced behaviors more slowly, have deficits in reversal learning demonstrating behavioral inflexibility, and exhibit reduced olfactory discrimination. When obese mice are removed from their high-fat diet to regain normal body weight and fasting glucose, olfactory dysfunctions are retained. We conclude that chronic energy imbalance therefore presents long-lasting structural and functional changes in the operation of the sensory system designed to encode external and internal chemical information and leads to altered olfactory- and reward-driven behaviors.
Keywords: chemical senses; circuitry; metabolism; obesity; olfactory; sensory.
Copyright © 2014 the authors 0270-6474/14/346970-15$15.00/0.
Figures


Comment in
-
Sensory systems: Eat well to improve smell?Nat Rev Neurosci. 2014 Jul;15(7):428. doi: 10.1038/nrn3771. Epub 2014 Jun 4. Nat Rev Neurosci. 2014. PMID: 24894584 No abstract available.
Similar articles
-
Consumption of dietary fat causes loss of olfactory sensory neurons and associated circuitry that is not mitigated by voluntary exercise in mice.J Physiol. 2022 Mar;600(6):1473-1495. doi: 10.1113/JP282112. Epub 2021 Dec 7. J Physiol. 2022. PMID: 34807463 Free PMC article.
-
Sensory systems: Eat well to improve smell?Nat Rev Neurosci. 2014 Jul;15(7):428. doi: 10.1038/nrn3771. Epub 2014 Jun 4. Nat Rev Neurosci. 2014. PMID: 24894584 No abstract available.
-
Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity.Physiol Behav. 2012 Oct 10;107(3):424-32. doi: 10.1016/j.physbeh.2012.09.007. Epub 2012 Sep 17. Physiol Behav. 2012. PMID: 22995978 Free PMC article.
-
Visualizing olfactory learning functional imaging of experience-induced olfactory bulb changes.Prog Brain Res. 2014;208:89-113. doi: 10.1016/B978-0-444-63350-7.00004-8. Prog Brain Res. 2014. PMID: 24767480 Review.
-
Subsystem organization of the mammalian sense of smell.Annu Rev Physiol. 2009;71:115-40. doi: 10.1146/annurev.physiol.70.113006.100608. Annu Rev Physiol. 2009. PMID: 18808328 Review.
Cited by
-
Western Diet Accelerates the Impairment of Odor-Related Learning and Olfactory Memory in the Mouse.ACS Chem Neurosci. 2020 Nov 4;11(21):3590-3602. doi: 10.1021/acschemneuro.0c00466. Epub 2020 Oct 15. ACS Chem Neurosci. 2020. PMID: 33054173 Free PMC article.
-
Olfactory and Gustatory Supra-Threshold Sensitivities Are Linked to Ad Libitum Snack Choice.Foods. 2022 Mar 10;11(6):799. doi: 10.3390/foods11060799. Foods. 2022. PMID: 35327222 Free PMC article.
-
Food odor perception promotes systemic lipid utilization.Nat Metab. 2022 Nov;4(11):1514-1531. doi: 10.1038/s42255-022-00673-y. Epub 2022 Nov 14. Nat Metab. 2022. PMID: 36376564
-
Differential Effects of BMI on Brain Response to Odor in Olfactory, Reward and Memory Regions: Evidence from fMRI.Nutrients. 2019 Apr 24;11(4):926. doi: 10.3390/nu11040926. Nutrients. 2019. PMID: 31022978 Free PMC article. Clinical Trial.
-
Consumption of dietary fat causes loss of olfactory sensory neurons and associated circuitry that is not mitigated by voluntary exercise in mice.J Physiol. 2022 Mar;600(6):1473-1495. doi: 10.1113/JP282112. Epub 2021 Dec 7. J Physiol. 2022. PMID: 34807463 Free PMC article.
References
-
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
