Memory accumulation mechanisms in human cortex are independent of motor intentions
- PMID: 24828652
- PMCID: PMC4019808
- DOI: 10.1523/JNEUROSCI.3911-13.2014
Memory accumulation mechanisms in human cortex are independent of motor intentions
Abstract
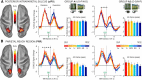
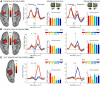
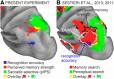
Previous studies on perceptual decision-making have often emphasized a tight link between decisions and motor intentions. Human decisions, however, also depend on memories or experiences that are not closely tied to specific motor responses. Recent neuroimaging findings have suggested that, during episodic retrieval, parietal activity reflects the accumulation of evidence for memory decisions. It is currently unknown, however, whether these evidence accumulation signals are functionally linked to signals for motor intentions coded in frontoparietal regions and whether activity in the putative memory accumulator tracks the amount of evidence for only previous experience, as reflected in "old" reports, or for both old and new decisions, as reflected in the accuracy of memory judgments. Here, human participants used saccadic-eye and hand-pointing movements to report recognition judgments on pictures defined by different degrees of evidence for old or new decisions. A set of cortical regions, including the middle intraparietal sulcus, showed a monotonic variation of the fMRI BOLD signal that scaled with perceived memory strength (older > newer), compatible with an asymmetrical memory accumulator. Another set, including the hippocampus and the angular gyrus, showed a nonmonotonic response profile tracking memory accuracy (higher > lower evidence), compatible with a symmetrical accumulator. In contrast, eye and hand effector-specific regions in frontoparietal cortex tracked motor intentions but were not modulated by the amount of evidence for the effector outcome. We conclude that item recognition decisions are supported by a combination of symmetrical and asymmetrical accumulation signals largely segregated from motor intentions.
Keywords: decision-making; diffusion models; effector specificity; episodic memory; fMRI; parietal lobe.
Copyright © 2014 the authors 0270-6474/14/346993-14$15.00/0.
Figures
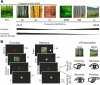

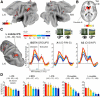
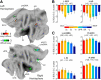
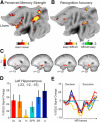
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
