Comparisons of interfacial Phe, Tyr, and Trp residues as determinants of orientation and dynamics for GWALP transmembrane peptides
- PMID: 24829070
- PMCID: PMC4053069
- DOI: 10.1021/bi500439x
Comparisons of interfacial Phe, Tyr, and Trp residues as determinants of orientation and dynamics for GWALP transmembrane peptides
Abstract
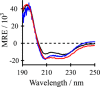
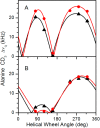
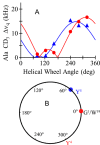
Aromatic amino acids often flank the transmembrane alpha helices of integral membrane proteins. By favoring locations within the membrane-water interface of the lipid bilayer, aromatic residues Trp, Tyr, and sometimes Phe may serve as anchors to help stabilize a transmembrane orientation. In this work, we compare the influence of interfacial Trp, Tyr, or Phe residues upon the properties of tilted helical transmembrane peptides. For such comparisons, it has been critical to start with no more than one interfacial aromatic residue near each end of a transmembrane helix, for example, that of GWALP23 (acetyl-GGALW(5)(LA)6LW(19)LAGA-[ethanol]amide). To this end, we have employed (2)H-labeled alanines and solid-state NMR spectroscopy to investigate the consequences of moving or replacing W5 or W19 in GWALP23 with selected Tyr, Phe, or Trp residues at the same or proximate locations. We find that GWALP23 peptides having F5, Y5, or W5 exhibit essentially the same average tilt and similar dynamics in bilayer membranes of 1,2-dilauroylphosphatidylcholine (DLPC) or 1,2-dioleoylphosphatidylcholine (DOPC). When double Tyr anchors are present, in Y(4,5)GWALP23 the NMR observables are markedly more subject to dynamic averaging and at the same time are less responsive to the bilayer thickness. Decreased dynamics are nevertheless observed when ring hydrogen bonding is removed, such that F(4,5)GWALP23 exhibits a similar extent of low dynamic averaging as GWALP23 itself. When F5 is the sole aromatic group in the N-interfacial region, the dynamic averaging is (only) slightly more extensive than with W5, Y5, or Y4 alone or with F4,5, yet it is much less than that observed for Y(4,5)GWALP23. Interestingly, moving Y5 to Y4 or W19 to W18, while retaining only one hydrogen-bond-capable aromatic ring at each interface, maintains the low level of dynamic averaging but alters the helix azimuthal rotation. The rotation change is about 40° for Y4 regardless of whether the host lipid bilayer is DLPC or DOPC. The rotational change (Δρ) is more dramatic and more complex when W19 is moved to W18, as Δρ is about +90° in DLPC but about -60° in DOPC. Possible reasons for this curious lipid-dependent helix rotation could include not only the separation distances between flanking aromatic or hydrophobic residues but also the absolute location of the W19 indole ring. For the more usual cases, when the helix azimuthal rotation shows little dependence on the host bilayer identity, excepting W(18)GWALP23, the transmembrane helices adapt to different lipids primarily by changing the magnitude of their tilt. We conclude that, in the absence of other functional groups, interfacial aromatic residues determine the preferred orientations and dynamics of membrane-spanning peptides. The results furthermore suggest possibilities for rotational and dynamic control of membrane protein function.
Figures




Similar articles
-
Tyrosine replacing tryptophan as an anchor in GWALP peptides.Biochemistry. 2012 Mar 13;51(10):2044-53. doi: 10.1021/bi201732e. Epub 2012 Mar 5. Biochemistry. 2012. PMID: 22364236 Free PMC article.
-
Single tryptophan and tyrosine comparisons in the N-terminal and C-terminal interface regions of transmembrane GWALP peptides.J Phys Chem B. 2013 Nov 7;117(44):13786-94. doi: 10.1021/jp407542e. Epub 2013 Oct 29. J Phys Chem B. 2013. PMID: 24111589 Free PMC article.
-
Response of GWALP transmembrane peptides to changes in the tryptophan anchor positions.Biochemistry. 2011 Sep 6;50(35):7522-35. doi: 10.1021/bi2006459. Epub 2011 Aug 12. Biochemistry. 2011. PMID: 21800919 Free PMC article.
-
Site-selective cleavage of peptides and proteins targeting aromatic amino acid residues.RSC Adv. 2025 Mar 25;15(12):9159-9179. doi: 10.1039/d4ra08956a. eCollection 2025 Mar 21. RSC Adv. 2025. PMID: 40134686 Free PMC article. Review.
-
NMR Studies of Aromatic Ring Flips to Probe Conformational Fluctuations in Proteins.J Phys Chem B. 2023 Jan 26;127(3):591-599. doi: 10.1021/acs.jpcb.2c07258. Epub 2023 Jan 14. J Phys Chem B. 2023. PMID: 36640108 Free PMC article. Review.
Cited by
-
Enhancing Antimicrobial Peptide Activity through Modifications of Charge, Hydrophobicity, and Structure.Int J Mol Sci. 2024 Oct 9;25(19):10821. doi: 10.3390/ijms251910821. Int J Mol Sci. 2024. PMID: 39409150 Free PMC article. Review.
-
Crystal structure and site-directed mutagenesis of circular bacteriocin plantacyclin B21AG reveals cationic and aromatic residues important for antimicrobial activity.Sci Rep. 2020 Oct 15;10(1):17398. doi: 10.1038/s41598-020-74332-1. Sci Rep. 2020. PMID: 33060678 Free PMC article.
-
Transmembrane Helix Integrity versus Fraying To Expose Hydrogen Bonds at a Membrane-Water Interface.Biochemistry. 2019 Feb 12;58(6):633-645. doi: 10.1021/acs.biochem.8b01119. Epub 2019 Jan 3. Biochemistry. 2019. PMID: 30565458 Free PMC article.
-
The Influence of Short Motifs on the Anticancer Activity of HB43 Peptide.Pharmaceutics. 2022 May 19;14(5):1089. doi: 10.3390/pharmaceutics14051089. Pharmaceutics. 2022. PMID: 35631675 Free PMC article.
-
Comparing Interfacial Trp, Interfacial His and pH Dependence for the Anchoring of Tilted Transmembrane Helical Peptides.Biomolecules. 2020 Feb 11;10(2):273. doi: 10.3390/biom10020273. Biomolecules. 2020. PMID: 32053887 Free PMC article.
References
-
- O’Connell A. M.; Koeppe R. E. II; Andersen O. S. (1990) Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science 250, 1256–1259. - PubMed
-
- Schiffer M.; Chang C. H.; Stevens F. J. (1992) The functions of tryptophan residues in membrane proteins. Protein Eng. 5, 213–214. - PubMed
-
- Landolt-Marticorena C.; Williams K. A.; Deber C. M.; Reithmeier R. A. (1993) Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 229, 602–608. - PubMed
-
- Killian J. A.; Salemink I.; de Planque M. R.; Lindblom G.; Koeppe R. E. II; Greathouse D. V. (1996) Induction of nonbilayer structures in diacylphosphatidylcholine model membranes by transmembrane alpha-helical peptides: importance of hydrophobic mismatch and proposed role of tryptophans. Biochemistry 35, 1037–1045. - PubMed
-
- de Planque M. R.; Kruijtzer J. A.; Liskamp R. M.; Marsh D.; Greathouse D. V.; Koeppe R. E. II; de Kruijff B.; Killian J. A. (1999) Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane alpha-helical peptides. J. Biol. Chem. 274, 20839–20846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

