6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children's Oncology Group study
- PMID: 24829202
- PMCID: PMC4192748
- DOI: 10.1182/blood-2014-01-552166
6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children's Oncology Group study
Abstract
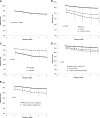
Durable remissions in children with acute lymphoblastic leukemia (ALL) require a 2-year maintenance phase that includes daily oral 6-mercaptopurine (6MP). Adherence to oral 6MP among Asian-American and African-American children with ALL is unknown. We enrolled 298 children with ALL (71 Asian Americans, 68 African Americans, and 159 non-Hispanic whites) receiving oral 6MP for the maintenance phase. Adherence was measured electronically for 39 803 person-days. Adherence declined from 95.0% (month 1) to 91.8% (month 5, P < .0001). Adherence rates were significantly (P < .0001) lower in Asian Americans (90.0% ± 4.9%) and African Americans (87.1% ± 4.4%), as compared with non-Hispanic whites (95.2% ± 1.3%). Race-specific sociodemographic characteristics helped explain poor adherence (African Americans: low maternal education [less than a college degree: 78.9%, vs at least college degree: 94.6%; P < .0001]; Asian Americans: low-income households [<$50 000: 84.5%, vs ≥$50 000: 96.7%; P = .04]; households without mothers as full-time caregivers [85.6%] vs households with mothers as full-time caregivers [97.2%; P = .05]). Adherence rate below 90% was associated with increased relapse risk (hazard ratio, 3.9; P = .01). Using an adherence rate <90% to define nonadherence, 20.5% of the participants were nonadherers. We identify race-specific determinants of adherence, and define a clinically relevant level of adherence needed to minimize relapse risk in a multiracial cohort of children with ALL. This trial was registered at www.clinicaltrials.gov as #NCT00268528.
© 2014 by The American Society of Hematology.
Figures

Comment in
-
It takes a village.Blood. 2014 Oct 9;124(15):2316-7. doi: 10.1182/blood-2014-05-576900. Blood. 2014. PMID: 25301330
References
-
- Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer. 2008;112(2):416–432. - PubMed
-
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. - PubMed
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14(1):18–24. - PubMed
-
- Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18(2):115–136. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R37 CA36401/CA/NCI NIH HHS/United States
- R37 CA036401/CA/NCI NIH HHS/United States
- U10 CA095861/CA/NCI NIH HHS/United States
- RC4 CA156449/CA/NCI NIH HHS/United States
- UG1 CA189955/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- GM 92666/GM/NIGMS NIH HHS/United States
- U01 GM092666/GM/NIGMS NIH HHS/United States
- CA 156449/CA/NCI NIH HHS/United States
- R01 CA096670/CA/NCI NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- U10 CA098543/CA/NCI NIH HHS/United States
- M01-RR00043/RR/NCRR NIH HHS/United States
- CA 21765/CA/NCI NIH HHS/United States
- U10 CA180886/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

