KLF1 mutations are relatively more common in a thalassemia endemic region and ameliorate the severity of β-thalassemia
- PMID: 24829204
- PMCID: PMC4118488
- DOI: 10.1182/blood-2014-03-561779
KLF1 mutations are relatively more common in a thalassemia endemic region and ameliorate the severity of β-thalassemia
Abstract
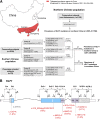
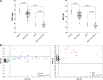
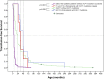
Mutations in human Krüppel-like factor 1 (KLF1) have recently been reported to be responsible for increased fetal hemoglobin (HbF) and hemoglobin A2 (HbA2). Because increased HbF and HbA2 levels are important features of β-thalassemia, we examined whether there is any relationship between KLF1 mutation and β-thalassemia in China. To do this, we first studied the incidence of KLF1 mutations in 2 Chinese populations: 3839 individuals from a thalassemia endemic region in south China and 1190 individuals from a non-thalassemia endemic region in north China. Interestingly, we found that the prevalence of KLF1 mutations is significantly higher in the thalassemia endemic region than that in non-thalassemia endemic region (1.25% vs 0.08%). Furthermore, we identified 7 functional variants including 4 previously reported (p.Gly176AlafsX179, p.Ala298Pro, p.Thr334Arg, and c.913+1G>A) and 3 novel variants (p.His299Asp, p.Cys341Tyr, and p.Glu5Lys) in southern China. The 2 most common mutations, p.Gly176AlafsX179 and p.His299Asp, accounted for 90.6% of the total. We found that zinc-finger mutations in KLF1 were selectively represented in 12 β-thalassemia intermedia patients and resulted in significantly different transfusion-free survival curves. Our findings suggest that KLF1 mutations occur selectively in the presence of β-thalassemia to increase the production of HbF, which in turn ameliorates the clinical severity of β-thalassemia.
© 2014 by The American Society of Hematology.
Figures
Comment in
-
KLF1: when less is more.Blood. 2014 Jul 31;124(5):672-3. doi: 10.1182/blood-2014-05-576967. Blood. 2014. PMID: 25082863 Free PMC article.
-
The Problem of Borderline Hemoglobin A2 Levels in the Screening for β-Thalassemia Carriers in Sardinia.Acta Haematol. 2016;135(4):193-9. doi: 10.1159/000442194. Epub 2016 Jan 22. Acta Haematol. 2016. PMID: 26794457
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

