Analysis of HLA A*02 association with vaccine efficacy in the RV144 HIV-1 vaccine trial
- PMID: 24829343
- PMCID: PMC4135964
- DOI: 10.1128/JVI.01164-14
Analysis of HLA A*02 association with vaccine efficacy in the RV144 HIV-1 vaccine trial
Abstract
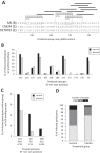
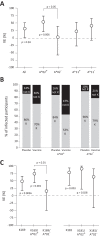
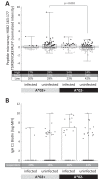
The RV144 HIV-1 vaccine trial demonstrated partial efficacy of 31% against HIV-1 infection. Studies into possible correlates of protection found that antibodies specific to the V1 and V2 (V1/V2) region of envelope correlated inversely with infection risk and that viruses isolated from trial participants contained genetic signatures of vaccine-induced pressure in the V1/V2 region. We explored the hypothesis that the genetic signatures in V1 and V2 could be partly attributed to selection by vaccine-primed T cells. We performed a T-cell-based sieve analysis of breakthrough viruses in the RV144 trial and found evidence of predicted HLA binding escape that was greater in vaccine versus placebo recipients. The predicted escape depended on class I HLA A*02- and A*11-restricted epitopes in the MN strain rgp120 vaccine immunogen. Though we hypothesized that this was indicative of postacquisition selection pressure, we also found that vaccine efficacy (VE) was greater in A*02-positive (A*02(+)) participants than in A*02(-) participants (VE = 54% versus 3%, P = 0.05). Vaccine efficacy against viruses with a lysine residue at site 169, important to antibody binding and implicated in vaccine-induced immune pressure, was also greater in A*02(+) participants (VE = 74% versus 15%, P = 0.02). Additionally, a reanalysis of vaccine-induced immune responses that focused on those that were shown to correlate with infection risk suggested that the humoral responses may have differed in A*02(+) participants. These exploratory and hypothesis-generating analyses indicate there may be an association between a class I HLA allele and vaccine efficacy, highlighting the importance of considering HLA alleles and host immune genetics in HIV vaccine trials.
Importance: The RV144 trial was the first to show efficacy against HIV-1 infection. Subsequently, much effort has been directed toward understanding the mechanisms of protection. Here, we conducted a T-cell-based sieve analysis, which compared the genetic sequences of viruses isolated from infected vaccine and placebo recipients. Though we hypothesized that the observed sieve effect indicated postacquisition T-cell selection, we also found that vaccine efficacy was greater for participants who expressed HLA A*02, an allele implicated in the sieve analysis. Though HLA alleles have been associated with disease progression and viral load in HIV-1 infection, these data are the first to suggest the association of a class I HLA allele and vaccine efficacy. While these statistical analyses do not provide mechanistic evidence of protection in RV144, they generate testable hypotheses for the HIV vaccine community and they highlight the importance of assessing the impact of host immune genetics in vaccine-induced immunity and protection. (This study has been registered at ClinicalTrials.gov under registration no. NCT00223080.).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Plotkin S, Orenstein W, Offit P. 2008. Vaccines, 5th ed. Elsevier Inc., Philadelphia, PA
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

