Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function
- PMID: 24829345
- PMCID: PMC4135960
- DOI: 10.1128/JVI.00724-14
Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function
Abstract
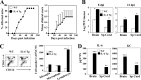
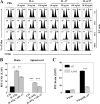
Interleukin-6 (IL-6) plays an important role in the development and progression of inflammatory responses, autoimmune diseases, and cancers. Many viral infections, including Theiler's murine encephalomyelitis virus (TMEV), result in the vigorous production of IL-6. However, the role of IL-6 in the development of virus-induced inflammatory responses is unclear. The infection of susceptible mice with TMEV induces the development of chronic demyelinating disease, which is considered a relevant infectious model for multiple sclerosis. In this study, we demonstrate that resistant C57BL/6 mice carrying an IL-6 transgene (IL-6 Tg) develop a TMEV-induced demyelinating disease accompanied by an increase in viral persistence and an elevated Th17 cell response in the central nervous system. Either IL-6 or IL-17 induced the expression of Bcl-2 and Bcl-xL at a high concentration. The upregulated expression of prosurvival molecules in turn inhibited target cell destruction by virus-specific CD8(+) T cells. More interestingly, IL-6 and IL-17 synergistically promoted the expression of these prosurvival molecules, preventing cellular apoptosis at a much lower (<5-fold) concentration. The signals involved in the synergy appear to include the activation of both STAT3 and NF-κB via distinct cytokine-dependent pathways. Thus, the excessive IL-6 promotes the generation of Th17 cells, and the resulting IL-6 and IL-17 synergistically promote viral persistence by protecting virus-infected cells from apoptosis and CD8(+) T cell-mediated target destruction. These results suggest that blocking both IL-6 and IL-17 functions are important considerations for therapies of chronic viral diseases, autoimmune diseases, and cancers.
Importance: This study indicates that an excessive level of IL-6 cytokine produced following viral infection promotes the development of IL-17-producing pathogenic helper T cells. We demonstrate here for the first time that IL-6 together with IL-17 synergistically enhances the expression of survival molecules to hinder critical host defense mechanisms removing virus-infected cells. This finding has an important implication in controlling not only chronic viral infections but also autoimmune diseases and cancers, which are associated with prolonged cell survival.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Critical role of TLR activation in viral replication, persistence, and pathogenicity of Theiler's virus.Front Immunol. 2023 Apr 20;14:1167972. doi: 10.3389/fimmu.2023.1167972. eCollection 2023. Front Immunol. 2023. PMID: 37153539 Free PMC article. Review.
-
Excessive Innate Immunity Steers Pathogenic Adaptive Immunity in the Development of Theiler's Virus-Induced Demyelinating Disease.Int J Mol Sci. 2021 May 17;22(10):5254. doi: 10.3390/ijms22105254. Int J Mol Sci. 2021. PMID: 34067536 Free PMC article. Review.
-
IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease.J Neuroinflammation. 2012 Sep 17;9:217. doi: 10.1186/1742-2094-9-217. J Neuroinflammation. 2012. PMID: 22985464 Free PMC article.
-
Viral hyperinfection of the central nervous system and high mortality after hematopoietic stem cell transplantation for treatment of Theiler's murine encephalomyelitis virus-induced demyelinating disease.Blood. 1999 Oct 15;94(8):2915-22. Blood. 1999. PMID: 10515897
-
Prostaglandin E2 produced following infection with Theiler's virus promotes the pathogenesis of demyelinating disease.PLoS One. 2017 Apr 26;12(4):e0176406. doi: 10.1371/journal.pone.0176406. eCollection 2017. PLoS One. 2017. PMID: 28445497 Free PMC article.
Cited by
-
The mechanism underlying extrapulmonary complications of the coronavirus disease 2019 and its therapeutic implication.Signal Transduct Target Ther. 2022 Feb 23;7(1):57. doi: 10.1038/s41392-022-00907-1. Signal Transduct Target Ther. 2022. PMID: 35197452 Free PMC article. Review.
-
Facets of Theiler's Murine Encephalomyelitis Virus-Induced Diseases: An Update.Int J Mol Sci. 2019 Jan 21;20(2):448. doi: 10.3390/ijms20020448. Int J Mol Sci. 2019. PMID: 30669615 Free PMC article. Review.
-
Cytokine Kinetics during Progression of COVID-19 in Rwanda Patients: Could IL-9/IFNγ Ratio Predict Disease Severity?Int J Mol Sci. 2023 Jul 31;24(15):12272. doi: 10.3390/ijms241512272. Int J Mol Sci. 2023. PMID: 37569646 Free PMC article.
-
COVID-19 infection: an overview on cytokine storm and related interventions.Virol J. 2022 May 26;19(1):92. doi: 10.1186/s12985-022-01814-1. Virol J. 2022. PMID: 35619180 Free PMC article. Review.
-
IL-6 Plays a Crucial Role in HBV Infection.J Clin Transl Hepatol. 2015 Dec 28;3(4):271-6. doi: 10.14218/JCTH.2015.00024. Epub 2015 Dec 15. J Clin Transl Hepatol. 2015. PMID: 26807383 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

