GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids
- PMID: 24829380
- PMCID: PMC4072574
- DOI: 10.1091/mbc.E13-12-0743
GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids
Abstract
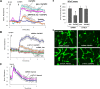
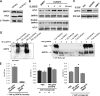
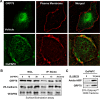
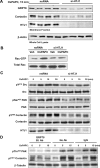
Vascular integrity and the maintenance of blood vessel continuity are fundamental features of the circulatory system maintained through endothelial cell-cell junctions. Defects in the endothelial barrier become an initiating factor in several pathologies, including ischemia/reperfusion, tumor angiogenesis, pulmonary edema, sepsis, and acute lung injury. Better understanding of mechanisms stimulating endothelial barrier enhancement may provide novel therapeutic strategies. We previously reported that oxidized phospholipids (oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine [OxPAPC]) promote endothelial cell (EC) barrier enhancement both in vitro and in vivo. This study examines the initiating mechanistic events triggered by OxPAPC to increase vascular integrity. Our data demonstrate that OxPAPC directly binds the cell membrane-localized chaperone protein, GRP78, associated with its cofactor, HTJ-1. OxPAPC binding to plasma membrane-localized GRP78 leads to GRP78 trafficking to caveolin-enriched microdomains (CEMs) on the cell surface and consequent activation of sphingosine 1-phosphate receptor 1, Src and Fyn tyrosine kinases, and Rac1 GTPase, processes essential for cytoskeletal reorganization and EC barrier enhancement. Using animal models of acute lung injury with vascular hyperpermeability, we observed that HTJ-1 knockdown blocked OxPAPC protection from interleukin-6 and ventilator-induced lung injury. Our data indicate for the first time an essential role of GRP78 and HTJ-1 in OxPAPC-mediated CEM dynamics and enhancement of vascular integrity.
© 2014 Birukova, Singleton, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




References
-
- Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007a;292:L924–L935. - PubMed
-
- Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol. 2007b;211:608–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

