mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia
- PMID: 24829408
- PMCID: PMC4128178
- DOI: 10.4049/jimmunol.1303492
mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia
Abstract
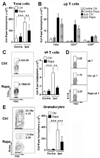
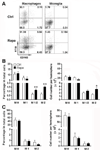
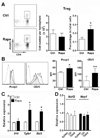
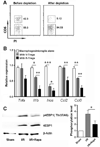
Signaling by the mammalian target of rapamycin (mTOR) plays an important role in the modulation of both innate and adaptive immune responses. However, the role and underlying mechanism of mTOR signaling in poststroke neuroinflammation are largely unexplored. In this study, we injected rapamycin, a mTOR inhibitor, by the intracerebroventricular route 6 h after focal ischemic stroke in rats. We found that rapamycin significantly reduced lesion volume and improved behavioral deficits. Notably, infiltration of γδ T cells and granulocytes, which are detrimental to the ischemic brain, was profoundly reduced after rapamycin treatment, as was the production of proinflammatory cytokines and chemokines by macrophages and microglia. Rapamycin treatment prevented brain macrophage polarization toward the M1 type. In addition, we also found that rapamycin significantly enhanced anti-inflammation activity of regulatory T cells (Tregs), which decreased production of proinflammatory cytokines and chemokines by macrophages and microglia. Depletion of Tregs partially elevated macrophage/microglia-induced neuroinflammation after stroke. Our data suggest that rapamycin can attenuate secondary injury and motor deficits after focal ischemia by enhancing the anti-inflammation activity of Tregs to restrain poststroke neuroinflammation.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. - PMC - PubMed
-
- Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–722. - PubMed
-
- Wang Y, Zhang Z, Chow N, Davis TP, Griffin JH, Chopp M, Zlokovic BV. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke; a journal of cerebral circulation. 2012;43:2444–2449. - PMC - PubMed
-
- Wechsler LR, Jovin TG. Intravenous recombinant tissue-type plasminogen activator in the extended time window and the US Food and Drug Administration: confused about the time. Stroke; a journal of cerebral circulation. 2012;43:2517–2519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

