Exosomes derived from Burkitt's lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells
- PMID: 24829410
- PMCID: PMC4174405
- DOI: 10.4049/jimmunol.1302068
Exosomes derived from Burkitt's lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells
Erratum in
-
Correction: Exosomes Derived from Burkitt's Lymphoma Cell Lines Induce Proliferation, Differentiation, and Class-Switch Recombination in B Cells.J Immunol. 2019 Aug 1;203(3):769-770. doi: 10.4049/jimmunol.1900638. Epub 2019 Jun 21. J Immunol. 2019. PMID: 31227578 No abstract available.
Abstract
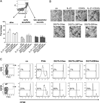
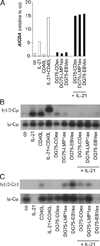
Exosomes, nano-sized membrane vesicles, are released by various cells and are found in many human body fluids. They are active players in intercellular communication and have immune-suppressive, immune-regulatory, and immune-stimulatory functions. EBV is a ubiquitous human herpesvirus that is associated with various lymphoid and epithelial malignancies. EBV infection of B cells in vitro induces the release of exosomes that harbor the viral latent membrane protein 1 (LMP1). LMP1 per se mimics CD40 signaling and induces proliferation of B lymphocytes and T cell-independent class-switch recombination. Constitutive LMP1 signaling within B cells is blunted through the shedding of LMP1 via exosomes. In this study, we investigated the functional effect of exosomes derived from the DG75 Burkitt's lymphoma cell line and its sublines (LMP1 transfected and EBV infected), with the hypothesis that they might mimic exosomes released during EBV-associated diseases. We show that exosomes released during primary EBV infection of B cells harbored LMP1, and similar levels were detected in exosomes from LMP1-transfected DG75 cells. DG75 exosomes efficiently bound to human B cells within PBMCs and were internalized by isolated B cells. In turn, this led to proliferation, induction of activation-induced cytidine deaminase, and the production of circle and germline transcripts for IgG1 in B cells. Finally, exosomes harboring LMP1 enhanced proliferation and drove B cell differentiation toward a plasmablast-like phenotype. In conclusion, our results suggest that exosomes released from EBV-infected B cells have a stimulatory capacity and interfere with the fate of human B cells.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures





Similar articles
-
Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23.J Virol. 1990 May;64(5):2309-18. doi: 10.1128/JVI.64.5.2309-2318.1990. J Virol. 1990. PMID: 2157887 Free PMC article.
-
LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1.Blood. 2008 Feb 1;111(3):1448-55. doi: 10.1182/blood-2007-10-117655. Epub 2007 Nov 15. Blood. 2008. PMID: 18006702
-
The Epstein-Barr virus latent membrane protein-1 (LMP1) induces interleukin-10 production in Burkitt lymphoma lines.Int J Cancer. 1994 Apr 15;57(2):240-4. doi: 10.1002/ijc.2910570218. Int J Cancer. 1994. PMID: 8157362
-
[Molecular biological properties of the Epstein-Barr virus LMP1 gene: structure, function and polymorphism].Vopr Virusol. 2015;60(3):5-13. Vopr Virusol. 2015. PMID: 26281300 Review. Russian.
-
Role of LMP1 in immune control of EBV infection.Semin Cancer Biol. 2001 Dec;11(6):455-60. doi: 10.1006/scbi.2001.0412. Semin Cancer Biol. 2001. PMID: 11669607 Review.
Cited by
-
Exosome-inflammasome crosstalk and their roles in inflammatory responses.Theranostics. 2021 Mar 4;11(9):4436-4451. doi: 10.7150/thno.54004. eCollection 2021. Theranostics. 2021. PMID: 33754070 Free PMC article. Review.
-
Hematological Malignancy-Derived Small Extracellular Vesicles and Tumor Microenvironment: The Art of Turning Foes into Friends.Cells. 2019 May 27;8(5):511. doi: 10.3390/cells8050511. Cells. 2019. PMID: 31137912 Free PMC article. Review.
-
Exosome Biogenesis, Regulation, and Function in Viral Infection.Viruses. 2015 Sep 17;7(9):5066-83. doi: 10.3390/v7092862. Viruses. 2015. PMID: 26393640 Free PMC article. Review.
-
Progress in Exosome Isolation Techniques.Theranostics. 2017 Jan 26;7(3):789-804. doi: 10.7150/thno.18133. eCollection 2017. Theranostics. 2017. PMID: 28255367 Free PMC article. Review.
-
Extracellular vesicles: potential roles in regenerative medicine.Front Immunol. 2014 Dec 3;5:608. doi: 10.3389/fimmu.2014.00608. eCollection 2014. Front Immunol. 2014. PMID: 25520717 Free PMC article. Review.
References
-
- Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. - PubMed
-
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73:1907–1920. - PubMed
-
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. - PubMed
-
- McLellan AD. Exosome release by primary B cells. Crit. Rev. Immunol. 2009;29:203–217. - PubMed
-
- Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin. Immunopathol. 2011;33:419–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

