IFN priming is necessary but not sufficient to turn on a migratory dendritic cell program in lupus monocytes
- PMID: 24829414
- PMCID: PMC5467885
- DOI: 10.4049/jimmunol.1301319
IFN priming is necessary but not sufficient to turn on a migratory dendritic cell program in lupus monocytes
Abstract
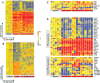
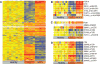
Blood monocytes from children with systemic lupus erythematosus (SLE) behave similar to dendritic cells (DCs), and SLE serum induces healthy monocytes to differentiate into DCs in a type I IFN-dependent manner. In this study, we found that these monocytes display significant transcriptional changes, including a prominent IFN signature, compared with healthy controls. Few of those changes, however, explain DC function. Exposure to allogeneic T cells in vitro reprograms SLE monocytes to acquire DC phenotype and function, and this correlates with both IFN-inducible (IP10) and proinflammatory cytokine (IL-1β and IL6) expression. Furthermore, we found that both IFN and SLE serum induce the upregulation of CCR7 transcription in these cells. CCR7 protein expression, however, requires a second signal provided by TLR agonists such as LPS. Thus, SLE serum "primes" a subset of monocytes to readily (<24 h) respond to TLR agonists and acquire migratory DC properties. Our findings might explain how microbial infections exacerbate lupus.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures



Similar articles
-
TLR4 ligands induce IFN-alpha production by mouse conventional dendritic cells and human monocytes after IFN-beta priming.J Immunol. 2009 Jan 15;182(2):820-8. doi: 10.4049/jimmunol.182.2.820. J Immunol. 2009. PMID: 19124725 Free PMC article.
-
Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus.J Immunol. 2006 Nov 1;177(9):5878-89. doi: 10.4049/jimmunol.177.9.5878. J Immunol. 2006. PMID: 17056512
-
A novel type I IFN-producing cell subset in murine lupus.J Immunol. 2008 Apr 1;180(7):5101-8. doi: 10.4049/jimmunol.180.7.5101. J Immunol. 2008. PMID: 18354236 Free PMC article.
-
Systemic lupus erythematosus: all roads lead to type I interferons.Curr Opin Immunol. 2006 Dec;18(6):676-82. doi: 10.1016/j.coi.2006.09.014. Epub 2006 Oct 2. Curr Opin Immunol. 2006. PMID: 17011763 Review.
-
A compass that points to lupus: genetic studies on type I interferon pathway.Genes Immun. 2007 Sep;8(6):445-55. doi: 10.1038/sj.gene.6364409. Epub 2007 Jun 21. Genes Immun. 2007. PMID: 17581625 Review.
Cited by
-
Elevated expression of miR-142-3p is related to the pro-inflammatory function of monocyte-derived dendritic cells in SLE.Arthritis Res Ther. 2016 Nov 16;18(1):263. doi: 10.1186/s13075-016-1158-z. Arthritis Res Ther. 2016. PMID: 27852285 Free PMC article.
-
Microarray expression profile of circular RNAs and mRNAs in children with systemic lupus erythematosus.Clin Rheumatol. 2019 May;38(5):1339-1350. doi: 10.1007/s10067-018-4392-8. Epub 2019 Jan 9. Clin Rheumatol. 2019. PMID: 30628013
-
Harnessing Mechanistic Knowledge on Beneficial Versus Deleterious IFN-I Effects to Design Innovative Immunotherapies Targeting Cytokine Activity to Specific Cell Types.Front Immunol. 2014 Oct 30;5:526. doi: 10.3389/fimmu.2014.00526. eCollection 2014. Front Immunol. 2014. PMID: 25400632 Free PMC article. Review.
-
Cell-cycle-gated feedback control mediates desensitization to interferon stimulation.Elife. 2020 Sep 18;9:e58825. doi: 10.7554/eLife.58825. Elife. 2020. PMID: 32945770 Free PMC article.
-
ATP1A1/BCL2L1 predicts the response of myelomonocytic and monocytic acute myeloid leukemia to cardiac glycosides.Leukemia. 2024 Jan;38(1):67-81. doi: 10.1038/s41375-023-02076-8. Epub 2023 Oct 30. Leukemia. 2024. PMID: 37904054 Free PMC article.
References
-
- Barber C, Gold WL, Fortin PR. Infections in the lupus patient: perspectives on prevention. Current opinion in rheumatology. 2011;23:358–365. - PubMed
-
- Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

