IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin
- PMID: 24829417
- PMCID: PMC4048788
- DOI: 10.4049/jimmunol.1301481
IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin
Abstract
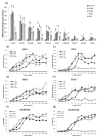
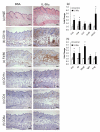
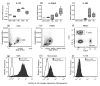
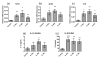
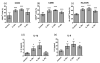
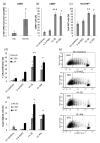
The IL-1 family members IL-36α (IL-1F6), IL-36β (IL-1F8), and IL-36γ (IL-1F9) and the receptor antagonist IL-36Ra (IL-1F5) constitute a novel signaling system that is poorly understood. We now show that these cytokines have profound effects on the skin immune system. Treatment of human keratinocytes with IL-36 cytokines significantly increased the expression of CXCL1, CXCL8, CCL3, CCL5, and CCL20, potent chemotactic agents for activated leukocytes, and IL-36α injected intradermally resulted in chemokine expression, leukocyte infiltration, and acanthosis of mouse skin. Blood monocytes, myeloid dendritic cells (mDC), and monocyte-derived DC (MO-DC) expressed IL-36R and responded to IL-36. In contrast, no direct effects of IL-36 on resting or activated human CD4(+) or CD8(+) T cells, or blood neutrophils, could be demonstrated. Monocytes expressed IL-1A, IL-1B, and IL-6 mRNA and IL-1β and IL-6 protein, and mDC upregulated surface expression of CD83, CD86, and HLA-DR and secretion of IL-1β and IL-6 after treatment with IL-36. Furthermore, IL-36α-treated MO-DC enhanced allogeneic CD4(+) T cell proliferation, demonstrating that IL-36 can stimulate the maturation and function of DC and drive T cell proliferation. These data indicate that IL-36 cytokines actively propagate skin inflammation via the activation of keratinocytes, APC, and, indirectly, T cells.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures
References
-
- Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 2004;279:13677–13688. - PubMed
-
- Debets R, Timans JC, Homey B, Zurawski S, Sana TR, Lo S, Wagner J, Edwards G, Clifford T, Menon S, Bazan JF, Kastelein RA. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–1446. - PubMed
-
- Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, Ward NL, Wohn C, Prens EP, Wang F, Maier LE, Kang S, Voorhees JJ, Elder JT, Gudjonsson JE. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

