Sphingolipids affect fibrinogen-induced caveolar transcytosis and cerebrovascular permeability
- PMID: 24829496
- PMCID: PMC4101621
- DOI: 10.1152/ajpcell.00305.2013
Sphingolipids affect fibrinogen-induced caveolar transcytosis and cerebrovascular permeability
Abstract
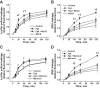
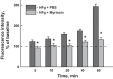
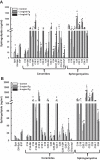
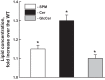
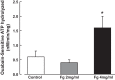
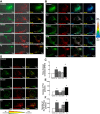
Inflammation-induced vascular endothelial dysfunction can allow plasma proteins to cross the vascular wall, causing edema. Proteins may traverse the vascular wall through two main pathways, the paracellular and transcellular transport pathways. Paracellular transport involves changes in endothelial cell junction proteins, while transcellular transport involves caveolar transcytosis. Since both processes are associated with filamentous actin formation, the two pathways are interconnected. Therefore, it is difficult to differentiate the prevailing role of one or the other pathway during various pathologies causing an increase in vascular permeability. Using a newly developed dual-tracer probing method, we differentiated transcellular from paracellular transport during hyperfibrinogenemia (HFg), an increase in fibrinogen (Fg) content. Roles of cholesterol and sphingolipids in formation of functional caveolae were assessed using a cholesterol chelator, methyl-β-cyclodextrin, and the de novo sphingolipid synthesis inhibitor myriocin. Fg-induced formation of functional caveolae was defined by association and colocalization of Na+-K+-ATPase and plasmalemmal vesicle-associated protein-1 with use of Förster resonance energy transfer and total internal reflection fluorescence microscopy, respectively. HFg increased permeability of the endothelial cell layer mainly through the transcellular pathway. While MβCD blocked Fg-increased transcellular and paracellular transport, myriocin affected only transcellular transport. Less pial venular leakage of albumin was observed in myriocin-treated HFg mice. HFg induced greater formation of functional caveolae, as indicated by colocalization of Na+-K+-ATPase with plasmalemmal vesicle-associated protein-1 by Förster resonance energy transfer and total internal reflection fluorescence microscopy. Our results suggest that elevated blood levels of Fg alter cerebrovascular permeability mainly by affecting caveolae-mediated transcytosis through modulation of de novo sphingolipid synthesis.
Keywords: Förster resonance energy transfer microscopy; cholesterol; functional caveolae; protein leakage; total internal reflection fluorescence microscopy.
Copyright © 2014 the American Physiological Society.
Figures
Similar articles
-
Cerebrovascular disorders caused by hyperfibrinogenaemia.J Physiol. 2016 Oct 15;594(20):5941-5957. doi: 10.1113/JP272558. Epub 2016 Jun 16. J Physiol. 2016. PMID: 27121987 Free PMC article.
-
Elevated level of fibrinogen increases caveolae formation; role of matrix metalloproteinase-9.Cell Biochem Biophys. 2014 Jun;69(2):283-94. doi: 10.1007/s12013-013-9797-z. Cell Biochem Biophys. 2014. PMID: 24307281 Free PMC article.
-
Role of caveolin-1 in the regulation of pulmonary endothelial permeability.Methods Mol Biol. 2011;763:303-17. doi: 10.1007/978-1-61779-191-8_21. Methods Mol Biol. 2011. PMID: 21874461
-
Lung Endothelial Transcytosis.Compr Physiol. 2020 Mar 12;10(2):491-508. doi: 10.1002/cphy.c190012. Compr Physiol. 2020. PMID: 32163197 Free PMC article. Review.
-
Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection.Viruses. 2018 Feb 8;10(2):69. doi: 10.3390/v10020069. Viruses. 2018. PMID: 29419739 Free PMC article. Review.
Cited by
-
Effects of fibrinogen synthesis inhibition on vascular cognitive impairment during traumatic brain injury in mice.Brain Res. 2021 Jan 15;1751:147208. doi: 10.1016/j.brainres.2020.147208. Epub 2020 Nov 26. Brain Res. 2021. PMID: 33248061 Free PMC article.
-
Fibrinogen-cellular prion protein complex formation on astrocytes.J Neurophysiol. 2020 Aug 1;124(2):536-543. doi: 10.1152/jn.00224.2020. Epub 2020 Jul 22. J Neurophysiol. 2020. PMID: 32697670 Free PMC article.
-
Fibrinogen and/or Fibrin as a Cause of Neuroinflammation.Online J Neurol Brain Disord. 2021;5(4):217. Epub 2021 Apr 14. Online J Neurol Brain Disord. 2021. PMID: 34327331 Free PMC article. No abstract available.
-
Fibrinogen and Neuroinflammation in the Neurovascular Unit in Stroke.J Inflamm Res. 2025 Apr 1;18:4567-4584. doi: 10.2147/JIR.S496433. eCollection 2025. J Inflamm Res. 2025. PMID: 40191094 Free PMC article. Review.
-
Cerebrovascular disorders caused by hyperfibrinogenaemia.J Physiol. 2016 Oct 15;594(20):5941-5957. doi: 10.1113/JP272558. Epub 2016 Jun 16. J Physiol. 2016. PMID: 27121987 Free PMC article.
References
-
- Abbruscato TJ, Lopez SP, Roder K, Paulson JR. Regulation of blood-brain barrier Na,K,2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. J Pharmacol Exp Ther 310: 459–468, 2004 - PubMed
-
- Ancellin N, Hla T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J Biol Chem 274: 18997–19002, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

