Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells
- PMID: 24829507
- PMCID: PMC4080154
- DOI: 10.1152/ajprenal.00251.2013
Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells
Abstract
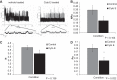
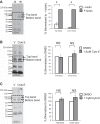
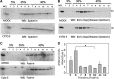
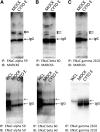
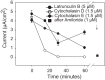
Numerous reports have linked cytoskeleton-associated proteins with the regulation of epithelial Na(+) channel (ENaC) activity. The purpose of the present study was to determine the effect of actin cytoskeleton disruption by cytochalasin E on ENaC activity in Xenopus 2F3 cells. Here, we show that cytochalasin E treatment for 60 min can disrupt the integrity of the actin cytoskeleton in cultured Xenopus 2F3 cells. We show using single channel patch-clamp experiments and measurements of short-circuit current that ENaC activity, but not its density, is altered by cytochalasin E-induced disruption of the cytoskeleton. In nontreated cells, 8 of 33 patches (24%) had no measurable ENaC activity, whereas in cytochalasin E-treated cells, 17 of 32 patches (53%) had no activity. Analysis of those patches that did contain ENaC activity showed channel open probability significantly decreased from 0.081 ± 0.01 in nontreated cells to 0.043 ± 0.01 in cells treated with cytochalasin E. Transepithelial current from mpkCCD cells treated with cytochalasin E, cytochalasin D, or latrunculin B for 60 min was decreased compared with vehicle-treated cells. The subcellular expression of fodrin changed significantly, and several protein elements of the cytoskeleton decreased at least twofold after 60 min of cytochalasin E treatment. Cytochalasin E treatment disrupted the association between ENaC and myristoylated alanine-rich C-kinase substrate. The results presented here suggest disruption of the actin cytoskeleton by different compounds can attenuate ENaC activity through a mechanism involving changes in the subcellular expression of fodrin, several elements of the cytoskeleton, and destabilization of the ENaC-myristoylated alanine-rich C-kinase substrate complex.
Keywords: actin; cytoskeleton; epithelial Na+ channels.
Copyright © 2014 the American Physiological Society.
Figures


Similar articles
-
Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane.Am J Physiol Renal Physiol. 2015 Sep 1;309(5):F456-63. doi: 10.1152/ajprenal.00631.2014. Epub 2015 Jul 1. Am J Physiol Renal Physiol. 2015. PMID: 26136560 Free PMC article.
-
ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins.Am J Physiol Cell Physiol. 2017 Jul 1;313(1):C42-C53. doi: 10.1152/ajpcell.00244.2016. Epub 2017 May 3. Am J Physiol Cell Physiol. 2017. PMID: 28468944 Free PMC article.
-
Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein.Am J Physiol Renal Physiol. 2012 Sep 15;303(6):F800-11. doi: 10.1152/ajprenal.00703.2011. Epub 2012 Jul 11. Am J Physiol Renal Physiol. 2012. PMID: 22791334 Free PMC article.
-
Intact cytoskeleton is required for small G protein dependent activation of the epithelial Na+ channel.PLoS One. 2010 Jan 21;5(1):e8827. doi: 10.1371/journal.pone.0008827. PLoS One. 2010. PMID: 20098689 Free PMC article.
-
Actin dynamics as critical ion channel regulator: ENaC and Piezo in focus.Am J Physiol Cell Physiol. 2021 May 1;320(5):C696-C702. doi: 10.1152/ajpcell.00368.2020. Epub 2021 Jan 20. Am J Physiol Cell Physiol. 2021. PMID: 33471624 Review.
Cited by
-
Direct and indirect inhibition of the circadian clock protein Per1: effects on ENaC and blood pressure.Am J Physiol Renal Physiol. 2019 May 1;316(5):F807-F813. doi: 10.1152/ajprenal.00408.2018. Epub 2019 Feb 13. Am J Physiol Renal Physiol. 2019. PMID: 30759025 Free PMC article.
-
The Pharmacological Inhibition of CaMKII Regulates Sodium Chloride Cotransporter Activity in mDCT15 Cells.Biology (Basel). 2021 Dec 16;10(12):1335. doi: 10.3390/biology10121335. Biology (Basel). 2021. PMID: 34943250 Free PMC article.
-
Ankyrin G Expression Regulates Apical Delivery of the Epithelial Sodium Channel (ENaC).J Biol Chem. 2017 Jan 6;292(1):375-385. doi: 10.1074/jbc.M116.753616. Epub 2016 Nov 28. J Biol Chem. 2017. PMID: 27895120 Free PMC article.
-
The diverse functions of the DEG/ENaC family: linking genetic and physiological insights.J Physiol. 2023 May;601(9):1521-1542. doi: 10.1113/JP283335. Epub 2022 Nov 13. J Physiol. 2023. PMID: 36314992 Free PMC article.
-
The Lectin-like Domain of TNF Increases ENaC Open Probability through a Novel Site at the Interface between the Second Transmembrane and C-terminal Domains of the α-Subunit.J Biol Chem. 2016 Nov 4;291(45):23440-23451. doi: 10.1074/jbc.M116.718163. Epub 2016 Sep 19. J Biol Chem. 2016. PMID: 27645999 Free PMC article.
References
-
- Alli AA, Gower WR., Jr The C type natriuretic peptide receptor tethers AHNAK1 at the plasma membrane to potentiate arachidonic acid-induced calcium mobilization. Am J Physiol Cell Physiol 297: C1157–C1167, 2009 - PubMed
-
- Alli AA, Gower WR., Jr Molecular approaches to examine the phosphorylation state of the C type natriuretic peptide receptor. J Cell Biochem 110: 985–994, 2010 - PubMed
-
- Assef YA, Ozu M, Marino GI, Galizia L, Kotsias BA. ENaC channels in oocytes from Xenopus laevis and their regulation by xShroom1 protein. Cell Physiol Biochem 28: 259–266, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

