Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells
- PMID: 24829507
- PMCID: PMC4080154
- DOI: 10.1152/ajprenal.00251.2013
Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells
Abstract
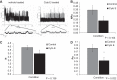
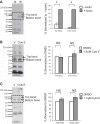
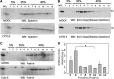
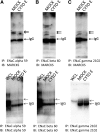
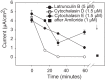
Numerous reports have linked cytoskeleton-associated proteins with the regulation of epithelial Na(+) channel (ENaC) activity. The purpose of the present study was to determine the effect of actin cytoskeleton disruption by cytochalasin E on ENaC activity in Xenopus 2F3 cells. Here, we show that cytochalasin E treatment for 60 min can disrupt the integrity of the actin cytoskeleton in cultured Xenopus 2F3 cells. We show using single channel patch-clamp experiments and measurements of short-circuit current that ENaC activity, but not its density, is altered by cytochalasin E-induced disruption of the cytoskeleton. In nontreated cells, 8 of 33 patches (24%) had no measurable ENaC activity, whereas in cytochalasin E-treated cells, 17 of 32 patches (53%) had no activity. Analysis of those patches that did contain ENaC activity showed channel open probability significantly decreased from 0.081 ± 0.01 in nontreated cells to 0.043 ± 0.01 in cells treated with cytochalasin E. Transepithelial current from mpkCCD cells treated with cytochalasin E, cytochalasin D, or latrunculin B for 60 min was decreased compared with vehicle-treated cells. The subcellular expression of fodrin changed significantly, and several protein elements of the cytoskeleton decreased at least twofold after 60 min of cytochalasin E treatment. Cytochalasin E treatment disrupted the association between ENaC and myristoylated alanine-rich C-kinase substrate. The results presented here suggest disruption of the actin cytoskeleton by different compounds can attenuate ENaC activity through a mechanism involving changes in the subcellular expression of fodrin, several elements of the cytoskeleton, and destabilization of the ENaC-myristoylated alanine-rich C-kinase substrate complex.
Keywords: actin; cytoskeleton; epithelial Na+ channels.
Copyright © 2014 the American Physiological Society.
Figures


References
-
- Alli AA, Gower WR., Jr The C type natriuretic peptide receptor tethers AHNAK1 at the plasma membrane to potentiate arachidonic acid-induced calcium mobilization. Am J Physiol Cell Physiol 297: C1157–C1167, 2009 - PubMed
-
- Alli AA, Gower WR., Jr Molecular approaches to examine the phosphorylation state of the C type natriuretic peptide receptor. J Cell Biochem 110: 985–994, 2010 - PubMed
-
- Assef YA, Ozu M, Marino GI, Galizia L, Kotsias BA. ENaC channels in oocytes from Xenopus laevis and their regulation by xShroom1 protein. Cell Physiol Biochem 28: 259–266, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

