Maintaining clarity: Review of maintenance therapy in non-small cell lung cancer
- PMID: 24829857
- PMCID: PMC4014782
- DOI: 10.5306/wjco.v5.i2.103
Maintaining clarity: Review of maintenance therapy in non-small cell lung cancer
Abstract
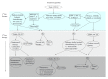
The purpose of this article is to review the role of maintenance therapy in the treatment of advanced non-small cell lung cancer (NSCLC). A brief overview about induction chemotherapy and its primary function in NSCLC is provided to address the basis of maintenance therapies foundation. The development of how maintenance therapy is utilized in this population is discussed and current guidelines for maintenance therapy are reviewed. Benefits and potential pitfalls of maintenance therapy are addressed, allowing a comprehensive review of the achieved clinical benefit that maintenance therapy may or may not have on NSCLC patient population. A review of current literature was conducted and a table is provided comparing the results of various maintenance therapy clinical trials. The table includes geographical location of each study, the number of patients enrolled, progression free survival and overall survival statistics, post-treatment regimens and if molecular testing was conducted. The role of molecular testing in relation to therapeutic treatment options for advanced NSCLC patients is discussed. A treatment algorithm clearly depicts first line and second line treatment for management of NSCLC and includes molecular testing, maintenance therapy and the role clinical trials have in treatment of NSCLC. This treatment algorithm has been specifically tailored and developed to assist clinicians in the management of advanced NSCLC.
Keywords: Clinical trials; Maintenance therapy; Molecular aberrations; Non-small cell lung cancer; Overall survival; Progression-free survival.
Figures
References
-
- World Health Organization. Cancer. 2013, cited 2013-10-30. Available from: http: //www.who.int/mediacentre/factsheets/fs297/en/
-
- Brodowicz T, Krzakowski M, Zwitter M, Tzekova V, Ramlau R, Ghilezan N, Ciuleanu T, Cucevic B, Gyurkovits K, Ulsperger E, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52:155–163. - PubMed
-
- Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W, Melezínek I, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. - PubMed
-
- National Comprehensive Cancer Network. Non Small Cell Lung Cancer. 2013, cited 2013-10-30. Available from: http: //www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
-
- Gridelli C, Ciardiello F, Gallo C, Feld R, Butts C, Gebbia V, Maione P, Morgillo F, Genestreti G, Favaretto A, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol. 2012;30:3002–3011. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

