Spermatid cyst polarization in Drosophila depends upon apkc and the CPEB family translational regulator orb2
- PMID: 24830287
- PMCID: PMC4022466
- DOI: 10.1371/journal.pgen.1004380
Spermatid cyst polarization in Drosophila depends upon apkc and the CPEB family translational regulator orb2
Abstract
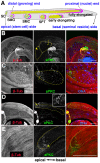
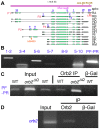
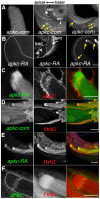
Mature Drosophila sperm are highly polarized cells--on one side is a nearly 2 mm long flagellar tail that comprises most of the cell, while on the other is the sperm head, which carries the gamete's genetic information. The polarization of the sperm cells commences after meiosis is complete and the 64-cell spermatid cyst begins the process of differentiation. The spermatid nuclei cluster to one side of the cyst, while the flagellar axonemes grows from the other. The elongating spermatid bundles are also polarized with respect to the main axis of the testis; the sperm heads are always oriented basally, while the growing tails extend apically. This orientation within the testes is important for transferring the mature sperm into the seminal vesicles. We show here that orienting cyst polarization with respect to the main axis of the testis depends upon atypical Protein Kinase C (aPKC), a factor implicated in polarity decisions in many different biological contexts. When apkc activity is compromised in the male germline, the direction of cyst polarization within this organ is randomized. Significantly, the mechanisms used to spatially restrict apkc activity to the apical side of the spermatid cyst are different from the canonical cross-regulatory interactions between this kinase and other cell polarity proteins that normally orchestrate polarization. We show that the asymmetric accumulation of aPKC protein in the cyst depends on an mRNA localization pathway that is regulated by the Drosophila CPEB protein Orb2. orb2 is required to properly localize and activate the translation of apkc mRNAs in polarizing spermatid cysts. We also show that orb2 functions not only in orienting cyst polarization with respect to the apical-basal axis of the testis, but also in the process of polarization itself. One of the orb2 targets in this process is its own mRNA. Moreover, the proper execution of this orb2 autoregulatory pathway depends upon apkc.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The 3'UTR of the Drosophila CPEB translation factor gene orb2 plays a crucial role in spermatogenesis.Development. 2021 Sep 1;148(17):dev198788. doi: 10.1242/dev.198788. Epub 2021 Sep 9. Development. 2021. PMID: 34473243 Free PMC article.
-
The CPEB protein Orb2 has multiple functions during spermatogenesis in Drosophila melanogaster.PLoS Genet. 2012;8(11):e1003079. doi: 10.1371/journal.pgen.1003079. Epub 2012 Nov 29. PLoS Genet. 2012. PMID: 23209437 Free PMC article.
-
The Drosophila CPEB protein Orb2 has a novel expression pattern and is important for asymmetric cell division and nervous system function.Genetics. 2011 Nov;189(3):907-21. doi: 10.1534/genetics.110.123646. Epub 2011 Sep 6. Genetics. 2011. PMID: 21900268 Free PMC article.
-
Establishing and maintaining cell polarity with mRNA localization in Drosophila.Bioessays. 2016 Mar;38(3):244-53. doi: 10.1002/bies.201500088. Epub 2016 Jan 15. Bioessays. 2016. PMID: 26773560 Free PMC article. Review.
-
Structure and molecular basis of spermatid elongation in the Drosophila testis.Open Biol. 2023 Nov;13(11):230136. doi: 10.1098/rsob.230136. Epub 2023 Nov 8. Open Biol. 2023. PMID: 37935354 Free PMC article. Review.
Cited by
-
The Physiological and Pathological Mechanisms of LIN2, LIN7, LIN10 and Their Tripartite Complex.J Cell Mol Med. 2025 Aug;29(15):e70794. doi: 10.1111/jcmm.70794. J Cell Mol Med. 2025. PMID: 40801824 Free PMC article. Review.
-
Subcellular Specialization and Organelle Behavior in Germ Cells.Genetics. 2018 Jan;208(1):19-51. doi: 10.1534/genetics.117.300184. Genetics. 2018. PMID: 29301947 Free PMC article. Review.
-
The 3'UTR of the Drosophila CPEB translation factor gene orb2 plays a crucial role in spermatogenesis.Development. 2021 Sep 1;148(17):dev198788. doi: 10.1242/dev.198788. Epub 2021 Sep 9. Development. 2021. PMID: 34473243 Free PMC article.
-
Long-Term Memory Formation in Drosophila Depends on the 3'UTR of CPEB Gene orb2.Cells. 2023 Jan 14;12(2):318. doi: 10.3390/cells12020318. Cells. 2023. PMID: 36672258 Free PMC article.
-
The CPEB ortholog Orb2 regulates brain size through the TRIM-NHL RNA-binding protein, Brain tumor.bioRxiv [Preprint]. 2025 Jul 22:2025.07.18.665534. doi: 10.1101/2025.07.18.665534. bioRxiv. 2025. PMID: 40777385 Free PMC article. Preprint.
References
-
- Etemad-Moghadam B, Guo S, Kemphues KJ (1995) Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83: 743–752. - PubMed
-
- Watts JL, Etemad-Moghadam B, Guo S, Boyd L, Draper BW, et al. (1996) par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development 122: 3133–3140. - PubMed
-
- Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, et al. (1998) Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development 125: 3607–3614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

