Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks' follow-up
- PMID: 24830290
- PMCID: PMC4022522
- DOI: 10.1371/journal.pone.0097482
Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks' follow-up
Abstract
Background: A comprehensive assessment of initial HIV-1 treatment success may inform study design and treatment guidelines.
Methods: Group-based, systematic review and meta-analysis of initial antiretroviral therapy studies, in adults, of ≥ 48 weeks duration, reported through December 31, 2012. Size-weighted, intention-to-treat efficacy was calculated. Parameters of study design/eligibility, participant and treatment characteristics were abstracted. Multivariable, random effects, linear regression models with backwards, stepwise selection were then used to identify variables associated with efficacy.
Outcome measures: Antiviral efficacy (undetectable plasma viral load) and premature cessation of therapy.
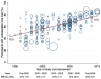
Results: 114 studies were included (216 treatment groups; 40,124 participants; mean CD4 count 248 cells/µL [SD 81]; mean HIV-1 plasma viral load log10 4.9 [SD 0.2]). Mean efficacy across all groups was 60% (SD 16) over a mean 82 weeks' follow-up (SD 38). Efficacy declined over time: 66% (SD 16) at 48 weeks, 60% (SD 16) at 96 weeks, 52% (SD 18) at 144 weeks. The most common reason for treatment cessation was participant decision (11%, SD 6.6). Efficacy was higher with 'Preferred' than 'Alternative' regimens (as defined by 2013 United States antiretroviral guidelines): 75% vs. 65%, respectively, difference 10%; 95%CI 7.6 to 15.4; p<0.001. In 98 groups (45%) reporting efficacy stratified by pre-treatment viral load (< or ≥100,000 copies/mL), efficacy was greater for the lower stratum (70% vs. 62%, respectively, difference 8.4%; 95%CI 6.0 to 10.9; p<0.001). This difference persisted within 'Preferred' regimens. Greatest efficacy was associated with use of tenofovir-emtricitabine (vs. other nucleoside analogue backbones) and integrase strand transfer inhibitors (vs. other third drug classes).
Conclusion: Initial antiretroviral treatments for HIV-1 to date appear to have suboptimal long-term efficacy, but are more effective when commenced at plasma viral loads <100,000 copies/mL. Rising viral load should be considered an indication for starting treatment. Integrase inhibitors offer a treatment advantage (vs. other third drug classes).
Conflict of interest statement
Figures


References
-
- Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Rockville, Maryland, USA: Department of Health and Human Services; 2013 (updated February 12).
-
- Bansi L, Sabin C, Gilson R, Gazzard B, Leen C, et al. (2009) Virological response to initial antiretroviral regimens containing abacavir or tenofovir. J Infect Dis 200: 710–714. - PubMed
-
- Bartlett JA, Fath MJ, Demasi R, Hermes A, Quinn J, et al. (2006) An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS 20: 2051–2064. - PubMed
-
- Carr A, Amin J (2009) Efficacy and tolerability of initial antiretroviral therapy: a systematic review. AIDS 23: 343–353. - PubMed
-
- Mbuagbaw LC, Irlam JH, Spaulding A, Rutherford GW, Siegfried N (2010) Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naive individuals. Cochrane Database Syst Rev: CD004246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

