Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway
- PMID: 24830293
- PMCID: PMC4022679
- DOI: 10.1371/journal.pone.0096760
Transcytosis of HIV-1 through vaginal epithelial cells is dependent on trafficking to the endocytic recycling pathway
Abstract
Background: While it is accepted that viruses can enter epithelial cells by endocytosis, the lack of an established biological mechanism for the trafficking of infectious virions through vaginal epithelial cells and their release from the plasma membrane has contributed to ongoing controversy about whether endocytosis is a mere artifact of some cell culture systems and whether squamous vaginal epithelial cells are even relevant as it pertains to HIV-1 transmission.
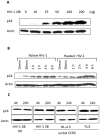
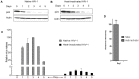
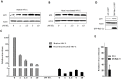
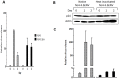
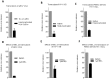
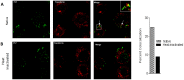
Methodology/principal findings: In this study, we investigated the intracellular trafficking pathway that HIV-1 exploits to transcytose vaginal epithelial cells. The reduction of endosome tubulation by recycling endosome inhibitors blocked transcytosis of HIV-1 in a cell culture and transwell system. In addition, we demonstrate that although heat-inactivated virus was endocytosed as efficiently as native virus, heat-inactivated virus was trafficked exclusively to the lysosomal pathway for degradation following endocytosis. Lysosomal protease-specific inhibitors blocked the degradation of inactivated virions. Immunofluorescence analysis not only demonstrated that HIV-1 was inside the cells but the different colocalization pattern of native vs. heat inactivated virus with transferrin provided conclusive evidence that HIV-1 uses the recycling pathway to get across vaginal epithelial cells.
Conclusions/significance: Altogether, our findings demonstrate the precise intracellular trafficking pathway utilized by HIV-1 in epithelial cells, confirms that HIV-1 transcytosis through vaginal epithelial cells is a biological phenomenon and brings to light the differential intracellular trafficking of native vs heat-inactivated HIV-1 which with further exploration could prove to provide valuable insights that could be used in the prevention of transcytosis/transmission of HIV-1 across the mucosal epithelia.
Conflict of interest statement
Figures

Similar articles
-
Endocytosed HIV-1 Envelope Glycoprotein Traffics to Rab14+ Late Endosomes and Lysosomes to Regulate Surface Levels in T-Cell Lines.J Virol. 2022 Jul 27;96(14):e0076722. doi: 10.1128/jvi.00767-22. Epub 2022 Jun 30. J Virol. 2022. PMID: 35770989 Free PMC article.
-
Oral and vaginal epithelial cell lines bind and transfer cell-free infectious HIV-1 to permissive cells but are not productively infected.PLoS One. 2014 May 23;9(5):e98077. doi: 10.1371/journal.pone.0098077. eCollection 2014. PLoS One. 2014. PMID: 24857971 Free PMC article.
-
Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells.J Virol. 2007 Jan;81(1):395-405. doi: 10.1128/JVI.01303-06. Epub 2006 Oct 18. J Virol. 2007. PMID: 17050597 Free PMC article.
-
Modeling mucosal cell-associated HIV type 1 transmission in vitro.J Infect Dis. 2014 Dec 15;210 Suppl 3(Suppl 3):S648-53. doi: 10.1093/infdis/jiu537. J Infect Dis. 2014. PMID: 25414419 Free PMC article. Review.
-
Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins.J Infect Dis. 1999 May;179 Suppl 3:S448-53. doi: 10.1086/314802. J Infect Dis. 1999. PMID: 10099117 Review.
Cited by
-
LAMP3/CD63 Expression in Early and Late Endosomes in Human Vaginal Epithelial Cells Is Associated with Enhancement of HSV-2 Infection.J Virol. 2022 Dec 14;96(23):e0155322. doi: 10.1128/jvi.01553-22. Epub 2022 Nov 9. J Virol. 2022. PMID: 36350153 Free PMC article.
-
Chlamydia trachomatis Infection of Endocervical Epithelial Cells Enhances Early HIV Transmission Events.PLoS One. 2016 Jan 5;11(1):e0146663. doi: 10.1371/journal.pone.0146663. eCollection 2016. PLoS One. 2016. PMID: 26730599 Free PMC article.
-
HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles.Virology. 2018 Feb;515:92-107. doi: 10.1016/j.virol.2017.12.012. Epub 2017 Dec 22. Virology. 2018. PMID: 29277006 Free PMC article.
-
Medroxyprogesterone Acetate (MPA) Enhances HIV-1 Accumulation and Release in Primary Cervical Epithelial Cells by Inhibiting Lysosomal Activity.Pathogens. 2021 Sep 14;10(9):1192. doi: 10.3390/pathogens10091192. Pathogens. 2021. PMID: 34578224 Free PMC article.
-
Encephalitic Alphaviruses Exploit Caveola-Mediated Transcytosis at the Blood-Brain Barrier for Central Nervous System Entry.mBio. 2020 Feb 11;11(1):e02731-19. doi: 10.1128/mBio.02731-19. mBio. 2020. PMID: 32047126 Free PMC article.
References
-
- Tuma P, Hubbard AL (2003) Transcytosis: crossing cellular barriers. Physiol Rev 83: 871–932. - PubMed
-
- Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132. - PubMed
-
- Bouschbacher M, Bomsel M, Verronese E, Gofflo S, Ganor Y, et al. (2008) Early events in HIV transmission through a human reconstructed vaginal mucosa. AIDS 22: 1257–1266. - PubMed
-
- Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P (2000) Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med 6: 475–479. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 HL007737/HL/NHLBI NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- G12 MD007586/MD/NIMHD NIH HHS/United States
- T32AI007281/AI/NIAID NIH HHS/United States
- SC1 GM089269/GM/NIGMS NIH HHS/United States
- U54 CA163066/CA/NCI NIH HHS/United States
- T32 AI007281/AI/NIAID NIH HHS/United States
- U54MD007593/MD/NIMHD NIH HHS/United States
- U54 MD007593/MD/NIMHD NIH HHS/United States
- R25 GM059994/GM/NIGMS NIH HHS/United States
- SC1GM089269/GM/NIGMS NIH HHS/United States
- T32HL007737/HL/NHLBI NIH HHS/United States
- P30AI054999/AI/NIAID NIH HHS/United States
- U54 RR026140/RR/NCRR NIH HHS/United States
- U54 CA163069/CA/NCI NIH HHS/United States
- UL1TR000445/TR/NCATS NIH HHS/United States
- U54 CA163072/CA/NCI NIH HHS/United States
- G12MD007586/MD/NIMHD NIH HHS/United States
- P30 AI054999/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

