Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx2
- PMID: 24830554
- PMCID: PMC4057745
- DOI: 10.3390/ijms15058509
Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx2
Abstract
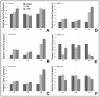
Freeze-drying is an effective means to control scaffold pore size and preserve its composition. The purpose of the present study was to determine the applicability of lyophilized Platelet-rich fibrin (LPRF) as a scaffold for craniofacial tissue regeneration and to compare its biological effects with commonly used fresh Platelet-rich fibrin (PRF). LPRF caused a 4.8-fold±0.4-fold elevation in Runt-related transcription factor 2 (Runx2) expression in alveolar bone cells, compared to a 3.6-fold±0.2-fold increase when using fresh PRF, and a more than 10-fold rise of alkaline phosphatase levels and mineralization markers. LPRF-induced Runx2 expression only occurred in alveolar bone and not in periodontal or dental follicle cells. LPRF also caused a 1.6-fold increase in osteoblast proliferation (p<0.001) when compared to fresh PRF. When applied in a rat craniofacial defect model for six weeks, LPRF resulted in 97% bony coverage of the defect, compared to 84% for fresh PRF, 64% for fibrin, and 16% without scaffold. Moreover, LPRF thickened the trabecular diameter by 25% when compared to fresh PRF and fibrin, and only LPRF and fresh PRF resulted in the formation of interconnected trabeculae across the defect. Together, these studies support the application of lyophilized PRF as a biomimetic scaffold for craniofacial bone regeneration and mineralized tissue engineering.
Figures





References
-
- Choukroun J., Diss A., Simonpieri A., Girard M.O., Schoeffler C., Dohan S.L., Dohan A.J., Mouhyi J., Dohan D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:e56–e60. - PubMed
-
- Bensaid W., Triffitt J.T., Blanchat C., Oudina K., Sedel L., Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–2502. - PubMed
-
- Kang Y.H., Jeon S.H., Park J.Y., Chung J.H., Choung Y.H., Choung H.W., Kim E.S., Choung P.H. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng. Part A. 2011;17:349–359. - PubMed
-
- He L., Lin Y., Hu X., Zhang Y., Wu H. A comparative study of Platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;108:707–713. - PubMed
-
- Dohan Ehrenfest D.M., Diss A., Odin G., Doglioli P., Hippolyte M.P., Charrier J.B. In vitro effects of Choukroun’s PRF (Platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;108:341–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

