Mathematical modeling reveals kinetics of lymphocyte recirculation in the whole organism
- PMID: 24830705
- PMCID: PMC4022467
- DOI: 10.1371/journal.pcbi.1003586
Mathematical modeling reveals kinetics of lymphocyte recirculation in the whole organism
Abstract
The kinetics of recirculation of naive lymphocytes in the body has important implications for the speed at which local infections are detected and controlled by immune responses. With a help of a novel mathematical model, we analyze experimental data on migration of 51Cr-labeled thoracic duct lymphocytes (TDLs) via major lymphoid and nonlymphoid tissues of rats in the absence of systemic antigenic stimulation. We show that at any point of time, 95% of lymphocytes in the blood travel via capillaries in the lung or sinusoids of the liver and only 5% migrate to secondary lymphoid tissues such as lymph nodes, Peyer's patches, or the spleen. Interestingly, our analysis suggests that lymphocytes travel via lung capillaries and liver sinusoids at an extremely rapid rate with the average residence time in these tissues being less than 1 minute. The model also predicts a relatively short average residence time of TDLs in the spleen (2.5 hours) and a longer average residence time of TDLs in major lymph nodes and Peyer's patches (10 hours). Surprisingly, we find that the average residence time of lymphocytes is similar in lymph nodes draining the skin (subcutaneous LNs) or the gut (mesenteric LNs) or in Peyer's patches. Applying our model to an additional dataset on lymphocyte migration via resting and antigen-stimulated lymph nodes we find that enlargement of antigen-stimulated lymph nodes occurs mainly due to increased entrance rate of TDLs into the nodes and not due to decreased exit rate as has been suggested in some studies. Taken together, our analysis for the first time provides a comprehensive, systems view of recirculation kinetics of thoracic duct lymphocytes in the whole organism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
 Cr] chromate. Labeled lymphocytes were passaged from blood to lymph in vivo by thoracic duct cannulation in an intermediate male. Collected lymphocytes were injected into female recipient rats and the percent of injected donor lymphocytes was measured in major lymphoid and nonlymphoid organs of the recipient rats. In the second set of experiments (cannulation data, panel B), TDLs were passaged via an intermediate host and then injected into the final recipients. Donor lymphocytes were counted during the thoracic duct cannulation of the recipient rats over the period of 45 hours.
Cr] chromate. Labeled lymphocytes were passaged from blood to lymph in vivo by thoracic duct cannulation in an intermediate male. Collected lymphocytes were injected into female recipient rats and the percent of injected donor lymphocytes was measured in major lymphoid and nonlymphoid organs of the recipient rats. In the second set of experiments (cannulation data, panel B), TDLs were passaged via an intermediate host and then injected into the final recipients. Donor lymphocytes were counted during the thoracic duct cannulation of the recipient rats over the period of 45 hours.
 where
where  and
and  . Cells leaving a particular organ return to the blood at a rate
. Cells leaving a particular organ return to the blood at a rate  with the exception of lymphocytes in the Peyer's patches from which lymphocytes migrate to the mesenteric LNs at a rate
with the exception of lymphocytes in the Peyer's patches from which lymphocytes migrate to the mesenteric LNs at a rate  [67, p. 470]. In these experiments the total number of labeled cells declined over time (Figure 2 in Text S1), and therefore we allow for a constant removal rate of TDLs from the blood
[67, p. 470]. In these experiments the total number of labeled cells declined over time (Figure 2 in Text S1), and therefore we allow for a constant removal rate of TDLs from the blood  occurring due to death and/or migration of lymphocytes to other tissues that were not sampled. The percent of transferred lymphocytes was measured in the blood (
occurring due to death and/or migration of lymphocytes to other tissues that were not sampled. The percent of transferred lymphocytes was measured in the blood ( ), lung (
), lung ( ), liver (
), liver ( ), spleen (
), spleen ( ), subcutaneous lymph nodes (SCLNs,
), subcutaneous lymph nodes (SCLNs,  ), mesenteric lymph nodes (MLNs,
), mesenteric lymph nodes (MLNs,  ), and Peyer's patches (PPs,
), and Peyer's patches (PPs,  ). Lymphocytes exiting lymph nodes return to the blood via right lymphatic and left lymphactic (thoracic) ducts (see also Figure 1 in Text S1). The thoracic duct collects lymph from all mesenteric lymph nodes and from approximately half (
). Lymphocytes exiting lymph nodes return to the blood via right lymphatic and left lymphactic (thoracic) ducts (see also Figure 1 in Text S1). The thoracic duct collects lymph from all mesenteric lymph nodes and from approximately half ( ) of subcutaneous lymph nodes . Lymph from other subcutaneous lymph nodes (
) of subcutaneous lymph nodes . Lymph from other subcutaneous lymph nodes ( ) enters the blood via the right lymphatic duct , .
) enters the blood via the right lymphatic duct , .
 Cr-labeled TDLs were passaged via an intermediate host and then were transferred into syngenic rats (Figure 1A). The percent of transferred cells was measured at different times after cell transfer in major lymphoid and nonlymphoid murine organs and is shown by markers. We fit the mathematical model of lymphocyte recirculation (eqn. (1) – (6)) to these experimental data using nonlinear least squares; model fits are shown as lines. Plots are for the first 30 minutes of the experiment (A) or for the whole experiment (B, abscissa values are plotted on the log-scale). Parameter estimates of the model are given in Table 1. Different y-scales in panels A and B were used for clarity.
Cr-labeled TDLs were passaged via an intermediate host and then were transferred into syngenic rats (Figure 1A). The percent of transferred cells was measured at different times after cell transfer in major lymphoid and nonlymphoid murine organs and is shown by markers. We fit the mathematical model of lymphocyte recirculation (eqn. (1) – (6)) to these experimental data using nonlinear least squares; model fits are shown as lines. Plots are for the first 30 minutes of the experiment (A) or for the whole experiment (B, abscissa values are plotted on the log-scale). Parameter estimates of the model are given in Table 1. Different y-scales in panels A and B were used for clarity.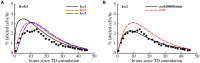
 Cr-labeled TDLs were passaged via an intermediate host and then transferred into final recipient rats. Recipients were cannulated via the thoracic duct (Figure 1B) and the rate of exit of labeled TDLs into the thoracic duct per hour was measured . The data are shown by markers (points). In panel A we show that for the parameter estimates from migration experiments (Table 1) the models with different number of subcompartments in LNs (
Cr-labeled TDLs were passaged via an intermediate host and then transferred into final recipient rats. Recipients were cannulated via the thoracic duct (Figure 1B) and the rate of exit of labeled TDLs into the thoracic duct per hour was measured . The data are shown by markers (points). In panel A we show that for the parameter estimates from migration experiments (Table 1) the models with different number of subcompartments in LNs ( ) fail to describe experimental data when
) fail to describe experimental data when  of lymphocytes exiting SCLNs migrate to the blood via the thoracic duct. To explain the data, we let the rate of lymphocyte exit from the LNs to decline exponentially with the time since cannulation,
of lymphocytes exiting SCLNs migrate to the blood via the thoracic duct. To explain the data, we let the rate of lymphocyte exit from the LNs to decline exponentially with the time since cannulation,  (panel B). We fit the data on the output rate of labeled cells into the thoracic duct using the mathematical model (eqn. (1) – (6)). We fix all model parameters to values shown in parameters for
(panel B). We fit the data on the output rate of labeled cells into the thoracic duct using the mathematical model (eqn. (1) – (6)). We fix all model parameters to values shown in parameters for  and fit only parameters
and fit only parameters  and
and  . The best description of the data was found when 1) the fraction
. The best description of the data was found when 1) the fraction  of lymphocytes in SCLNs enter the blood via the thoracic (left lymphatic) duct, and 2) the rate of lymphocyte migration via lymph nodes and Peyer's patches declines with time since cannulation at a rate
of lymphocytes in SCLNs enter the blood via the thoracic (left lymphatic) duct, and 2) the rate of lymphocyte migration via lymph nodes and Peyer's patches declines with time since cannulation at a rate  min
min (solid line in panel B). The model fails to predict thoracic duct output data if residence times of lymphocytes in LNs is unaffected by cannulation (
(solid line in panel B). The model fails to predict thoracic duct output data if residence times of lymphocytes in LNs is unaffected by cannulation ( , large dashing lines in panels A and B).
, large dashing lines in panels A and B).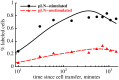
 min
min and
and  min
min , respectively. Exit rate of lymphocytes from antigen-stimulated and unstimulated pLNs are
, respectively. Exit rate of lymphocytes from antigen-stimulated and unstimulated pLNs are  min
min and
and  min
min (two sub-compartments in the LNs,
(two sub-compartments in the LNs,  ). To describe the dynamics of TDLs in the blood and other organs we used parameters given in Table 1. Our results suggest that antigenic stimulation of the lymph node with sheep erythrocytes increases the rate of entrance of TDLs into the LN almost 4 fold without a significant change in the lymphocyte exit rate (
). To describe the dynamics of TDLs in the blood and other organs we used parameters given in Table 1. Our results suggest that antigenic stimulation of the lymph node with sheep erythrocytes increases the rate of entrance of TDLs into the LN almost 4 fold without a significant change in the lymphocyte exit rate ( ,
,  ).
).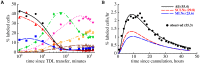
 subcompartments in LNs and PPs (see Materials and Methods). In panel A, symbol and line labeling is similar to that of Figure 3. The model predicts that the
subcompartments in LNs and PPs (see Materials and Methods). In panel A, symbol and line labeling is similar to that of Figure 3. The model predicts that the  of lymphocytes exiting SCLNs migrate to the blood via the right lymphatic duct and thus are not sampled during the thoracic duct cannulation. Numbers in parentheses in panel B indicate the percent of cells exiting into blood from different LNs in 45 hours as predicted by the model (lines) or as observed in the data (dots). In the data, 55% of transferred TDLs were collected during 45 hours of the thoracic duct cannulation. The model also predicts the contribution of lymphocytes exiting SCLNs (short dashed line in panel B) and MLNs (long dashed line in panel B) via the thoracic duct. To explain the data, the rate of egress of lymphocytes from LNs and PPs declines exponentially with time during cannulation at an estimated rate
of lymphocytes exiting SCLNs migrate to the blood via the right lymphatic duct and thus are not sampled during the thoracic duct cannulation. Numbers in parentheses in panel B indicate the percent of cells exiting into blood from different LNs in 45 hours as predicted by the model (lines) or as observed in the data (dots). In the data, 55% of transferred TDLs were collected during 45 hours of the thoracic duct cannulation. The model also predicts the contribution of lymphocytes exiting SCLNs (short dashed line in panel B) and MLNs (long dashed line in panel B) via the thoracic duct. To explain the data, the rate of egress of lymphocytes from LNs and PPs declines exponentially with time during cannulation at an estimated rate  min
min . Other parameters for the migration kinetics of TDLs are nearly identical to those given in Table 1. We fixed
. Other parameters for the migration kinetics of TDLs are nearly identical to those given in Table 1. We fixed  in fits of these data. Estimated standard errors are
in fits of these data. Estimated standard errors are  and
and  (see eqn. (8)).
(see eqn. (8)).Similar articles
-
Estimating Residence Times of Lymphocytes in Ovine Lymph Nodes.Front Immunol. 2019 Jul 16;10:1492. doi: 10.3389/fimmu.2019.01492. eCollection 2019. Front Immunol. 2019. PMID: 31379805 Free PMC article.
-
Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo.J Immunol. 1991 Dec 15;147(12):4178-84. J Immunol. 1991. PMID: 1753094
-
Evidence of extensive lymphocyte death in sheep Peyer's patches. II. The number and fate of newly-formed lymphocytes that emigrate from Peyer's patches.J Immunol. 1986 Mar 15;136(6):2011-7. J Immunol. 1986. PMID: 3950408
-
The function and pathways of lymphocyte recirculation.Ciba Found Symp. 1980;71:113-26. doi: 10.1002/9780470720547.ch7. Ciba Found Symp. 1980. PMID: 6989563 Review.
-
Specific lymphocyte-endothelial cell interactions regulate migration into lymph nodes, Peyer's patches, and skin.Reg Immunol. 1988 Jul-Aug;1(1):78-83. Reg Immunol. 1988. PMID: 3079311 Review.
Cited by
-
Analysis of cellular kinetic models suggest that physiologically based model parameters may be inherently, practically unidentifiable.J Pharmacokinet Pharmacodyn. 2022 Oct;49(5):539-556. doi: 10.1007/s10928-022-09819-7. Epub 2022 Aug 6. J Pharmacokinet Pharmacodyn. 2022. PMID: 35933452 Free PMC article.
-
Transport Barriers Influence the Activation of Anti-Tumor Immunity: A Systems Biology Analysis.Adv Sci (Weinh). 2023 Dec;10(36):e2304076. doi: 10.1002/advs.202304076. Epub 2023 Nov 10. Adv Sci (Weinh). 2023. PMID: 37949675 Free PMC article.
-
Tissue-Resident Memory T Cells in Mice and Humans: Towards a Quantitative Ecology.J Immunol. 2019 Nov 15;203(10):2561-2569. doi: 10.4049/jimmunol.1900767. J Immunol. 2019. PMID: 31685700 Free PMC article. Review.
-
A multicompartment mathematical model based on host immunity for dissecting COVID-19 heterogeneity.Heliyon. 2022 May;8(5):e09488. doi: 10.1016/j.heliyon.2022.e09488. Epub 2022 May 18. Heliyon. 2022. PMID: 35600458 Free PMC article.
-
Lymphatic Dissemination of Simian Immunodeficiency Virus after Penile Inoculation.J Virol. 2016 Mar 28;90(8):4093-4104. doi: 10.1128/JVI.02947-15. Print 2016 Apr. J Virol. 2016. PMID: 26865706 Free PMC article.
References
-
- Ford, W. & Gowans (1969) J (1969) The traffic of lymphocytes. Semin Hematol 6(1): 67–83. - PubMed
-
- Westermann, J., Ehlers, E., Exton, M., Kaiser, M. & Bode, U. 2001 Migration of naive, effector and memory T cells: implications for the regulation of immune responses. Immunol Rev 184: 20–37. - PubMed
-
- Westermann J, Persin S, Matyas J, van der Meide, P. & Pabst (1994) R (1994) Migration of so-called naive and memory T lymphocytes from blood to lymph in the rat. The influence of IFN-gamma on the circulation pattern. J Immunol 152(4): 1744–1750. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

