E-cigarette versus nicotine inhaler: comparing the perceptions and experiences of inhaled nicotine devices
- PMID: 24830741
- PMCID: PMC4238186
- DOI: 10.1007/s11606-014-2889-7
E-cigarette versus nicotine inhaler: comparing the perceptions and experiences of inhaled nicotine devices
Abstract
Background: Novel nicotine delivery products, such as electronic cigarettes (e-cigarettes), have dramatically grown in popularity despite limited data on safety and benefit. In contrast, the similar U.S. Food and Drug Administration (FDA)-approved nicotine inhaler is rarely utilized by smokers. Understanding this paradox could be helpful to determine the potential for e-cigarettes as an alternative to tobacco smoking.
Objective: To compare the e-cigarette with the nicotine inhaler in terms of perceived benefits, harms, appeal, and role in assisting with smoking cessation.
Design: A cross-over trial was conducted from 2012 to 2013 PARTICIPANTS/INTERVENTIONS: Forty-one current smokers age 18 and older used the e-cigarette and nicotine inhaler each for 3 days, in random order, with a washout period in between. Thirty-eight participants provided data on product use, perceptions, and experiences.
Main measures: The Modified Cigarette Evaluation Questionnaire (mCEQ) measured satisfaction, reward, and aversion. Subjects were also asked about each product's helpfulness, similarity to cigarettes, acceptability, image, and effectiveness in quitting smoking. Cigarette use was also recorded during the product-use periods.
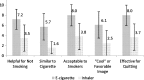
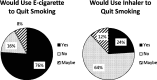
Key results: The e-cigarette had a higher total satisfaction score (13.9 vs. 6.8 [p < 0.001]; range for responses 3-21) and higher reward score (15.8 vs. 8.7 [p < 0.001]; range for responses 5-35) than the inhaler. The e-cigarette received higher ratings for helpfulness, acceptability, and "coolness." More subjects would use the e-cigarette to make a quit attempt (76 %) than the inhaler (24 %) (p < 0.001). Eighteen percent (7/38) of subjects abstained from smoking during the 3-day periods using the e-cigarette vs. 10 % (4/38) using the inhaler (p = 0.18).
Conclusion: The e-cigarette was more acceptable, provided more satisfaction, and had higher perceived benefit than the inhaler during this trial. E-cigarettes have the potential to be important nicotine delivery products owing to their high acceptance and perceived benefit, but more data are needed to evaluate their actual efficacy and safety. Providers should be aware of these issues, as patients will increasingly inquire about them.
Figures
Comment in
-
What should we tell our patients about e-cigarettes?J Gen Intern Med. 2014 Nov;29(11):1427-8. doi: 10.1007/s11606-014-2943-5. J Gen Intern Med. 2014. PMID: 25005617 Free PMC article. No abstract available.
References
-
- Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville: U.S. Department of Health and Human Services. Public Health Service; 2008.
-
- Steinberg MB, Evans RM, Hughes JR, Leone FT, Lipsky M. Treatment of Tobacco Dependence; AMA Therapeutic Insights; 2011. Available at http://www.ama-assn.org/ama/pub/education-careers/continuing-medical-edu... (accessed 25 April 2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical