The double zinc finger domain and adjacent accessory domain from the transcription factor loss of zinc sensing 1 (loz1) are necessary for DNA binding and zinc sensing
- PMID: 24831008
- PMCID: PMC4140304
- DOI: 10.1074/jbc.M114.551333
The double zinc finger domain and adjacent accessory domain from the transcription factor loss of zinc sensing 1 (loz1) are necessary for DNA binding and zinc sensing
Abstract
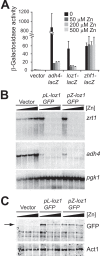
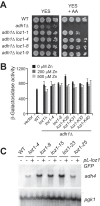
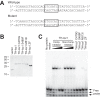
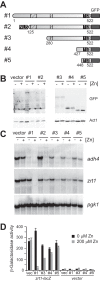
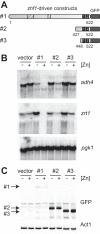
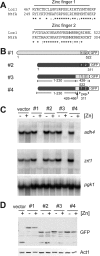
The Loz1 transcription factor from Schizosaccharomyces pombe plays an essential role in zinc homeostasis by repressing target gene expression in zinc-replete cells. To determine how Loz1 function is regulated by zinc, we employed a genetic screen to isolate mutants with impaired zinc-dependent gene expression and analyzed Loz1 protein truncations to map a minimal zinc-responsive domain. In the screen, we isolated 36 new loz1 alleles. 27 of these alleles contained mutations resulting in the truncation of the Loz1 protein. The remaining nine alleles contained point mutations leading to an amino acid substitution within a C-terminal double zinc finger domain. Further analysis of two of these substitutions revealed that they disrupted Loz1 DNA activity in vitro. By analyzing Loz1 protein truncations, we found that the last 96 amino acids of Loz1 was the smallest region that was able to confer partial zinc-dependent repression in vivo. This 96-amino acid region contains the double zinc finger domain and an accessory domain that enhances DNA binding. These results were further supported by the findings that MtfA, a transcription factor from Aspergillus nidulans that contains a related double zinc finger, is unable to complement loz1Δ, whereas a chimera of MtfA containing the Loz1 accessory domain is able to complement loz1Δ. Together, our studies indicate that the double zinc finger domain and adjacent accessory domain preceding zinc finger 1 are necessary for DNA binding and zinc-dependent repression.
Keywords: Metal Homeostasis; Metallosensor; Transcription Repressor; Yeast Genetics; Zinc; Zinc Finger.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Andreini C., Bertini I. (2012) A bioinformatics view of zinc enzymes. J. Inorg. Biochem. 111, 150–156 - PubMed
-
- Klug A. (2010) The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu. Rev. Biochem. 79, 213–231 - PubMed
-
- Ehrensberger K. M., Bird A. J. (2011) Hammering out details: regulating metal levels in eukaryotes. Trends Biochem. Sci. 36, 524–531 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

