The zinc finger transcription factor ZFX is required for maintaining the tumorigenic potential of glioblastoma stem cells
- PMID: 24831540
- PMCID: PMC4349564
- DOI: 10.1002/stem.1730
The zinc finger transcription factor ZFX is required for maintaining the tumorigenic potential of glioblastoma stem cells
Abstract
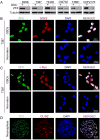
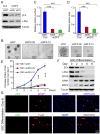
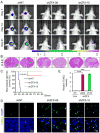
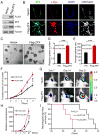
Glioblastomas are highly lethal brain tumors containing tumor-propagating glioma stem cells (GSCs). The molecular mechanisms underlying the maintenance of the GSC phenotype are not fully defined. Here we demonstrate that the zinc finger and X-linked transcription factor (ZFX) maintains GSC self-renewal and tumorigenic potential by upregulating c-Myc expression. ZFX is differentially expressed in GSCs relative to non-stem glioma cells and neural progenitor cells. Disrupting ZFX by shRNA reduced c-Myc expression and potently inhibited GSC self-renewal and tumor growth. Ectopic expression of c-Myc to its endogenous level rescued the effects caused by ZFX disruption, supporting that ZFX controls GSC properties through c-Myc. Furthermore, ZFX binds to a specific sequence (GGGCCCCG) on the human c-Myc promoter to upregulate c-Myc expression. These data demonstrate that ZFX functions as a critical upstream regulator of c-Myc and plays essential roles in the maintenance of the GSC phenotype. This study also supports that c-Myc is a dominant driver linking self-renewal to malignancy.
Keywords: Cancer stem cell; Glioblastoma; Self-renewal; Tumorigenesis; Zinc finger and X-linked transcription factor; c-Myc.
© 2014 AlphaMed Press.
Conflict of interest statement
The authors declare no potential conflicts of interests.
Figures


References
-
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

